Purity Assessment and Dynamic Monitoring of the Viral Genome Release Process
Quality control is indispensable to ensure the adequate purity of virus products in the biopharmaceutical industry. Purity assessment of virus products is crucial in many biotechnology applications. Many particles can coexist in the lysates of the host cells infected with a wild-type phage, including cell debris, DNA-free proheads, empty capsids (after DNA ejection), and mature virions. Meanwhile, the natural process of viruses delivering their genes into the hosts for self-replication has inspired the design of virus-like particles to deliver therapeutic or imaging agents. Monitoring the viral genome release process will allow for deeper insights into the mechanisms of cargo release. The Flow NanoAnalyzer is applied to analyze the composition of the viral sample and monitor the viral genome release process following treatment with NaClO4.
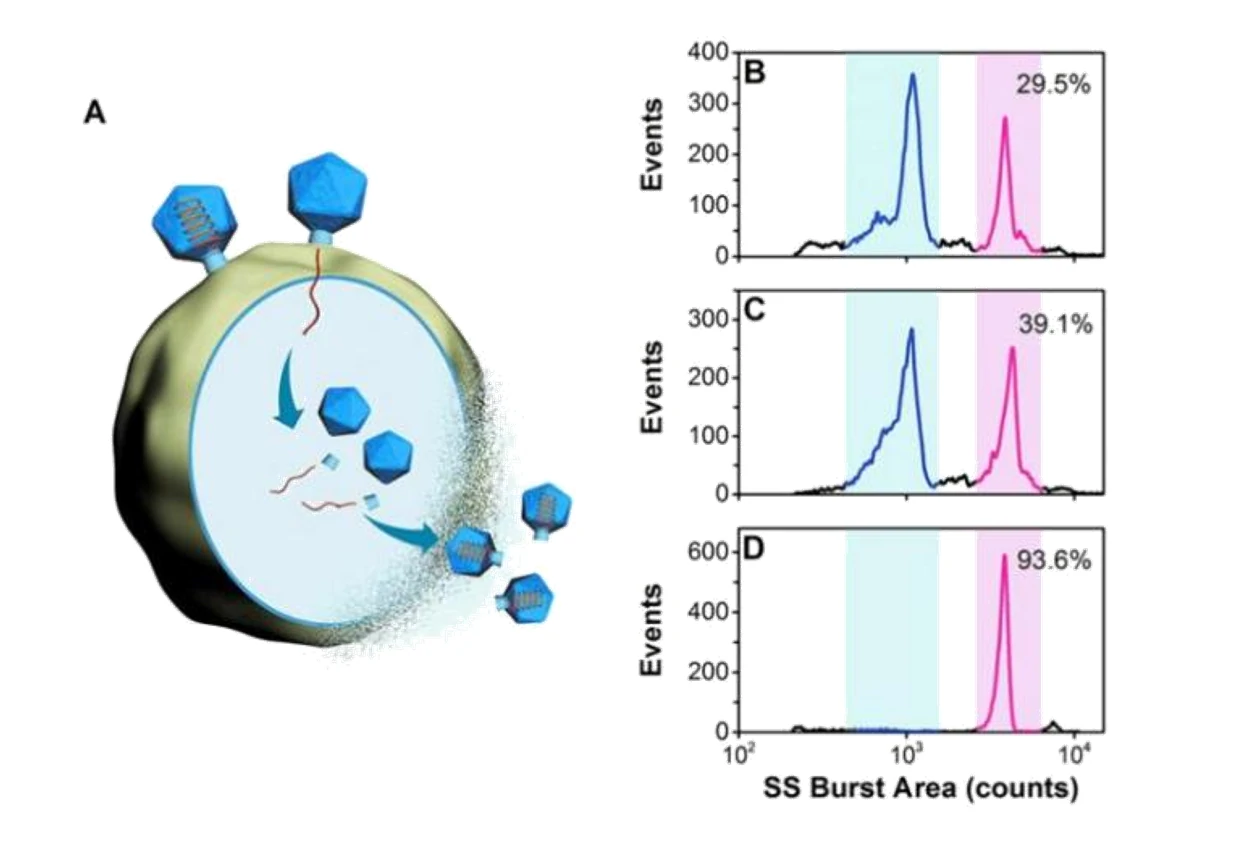
Figure 1. Analysis of crude samples for bacteriophage T7 from different batches.
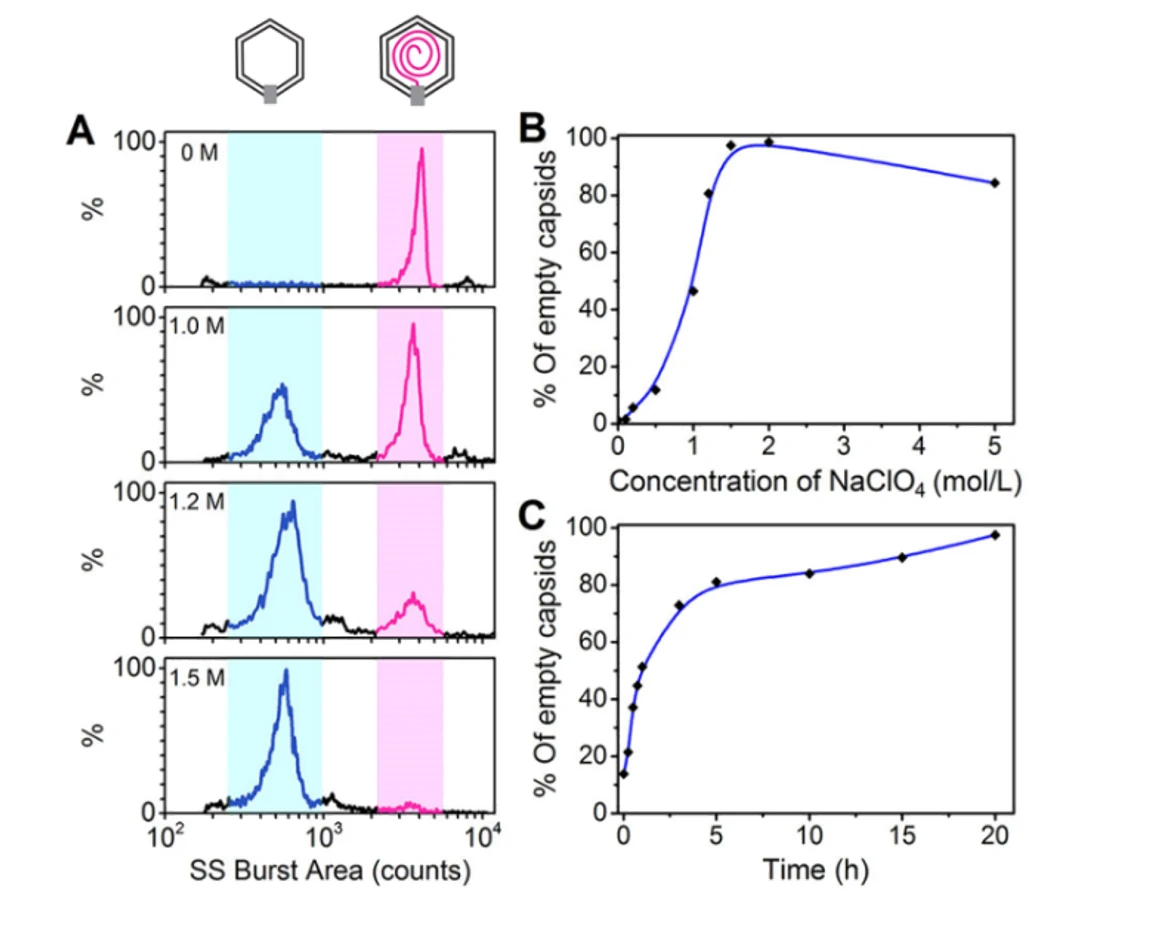
Figure 2. Analysis of NaClO4-triggered DNA ejection process of bacteriophage T7
The differentiation of capsids before DNA packaging (proheads, ~1100 counts) and after DNA release (empty capsids, ~600 counts), indicates that the Flow NanoAnalyzer could be an efficient tool for virology study.
Angew. Chem. Int. Edit., 2016, 55(35), 10239-10243.




