MITOCHONDRIA
HIGH-THROUGHPUT MULTIPARAMETER ANALYSIS OF INDIVIDUAL MITOCHONDRIA
1. Estimation of Purity and Structural Integrity of Single Mitochondria
IntroductionMitochondria play a central role in the regulation of energy metabolism and signal transduction in eukaryotic cells. Mitochondrial dynamics actively contributes to a lot of cellular activities, such as mitophagy, apoptosis and natural immunity. Mitochondrial dysfunction may lead to several human diseases, including neurodegenerative diseases and metabolic disturbance. Therefore, mitochondrial study is of great significance for basic life science development. However, conventional flow cytometric analysis of individual mitochondria has been limited to attributes that can be brightly stained.
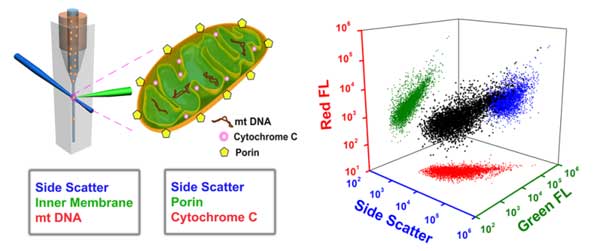
Here the Flow NanoAnalyzer is applied for the high-throughput multiparameter analysis of individual mitochondria. By simultaneous labeling mitochondrial cardiolipin (inner membrane) and mtDNA (matrix) with NAO and SYTO 62, respectively, Flow NanoAnalyzer demonstrates the assessment of the purity and structural integrity of individual mitochondria. By simultaneous labeling of porin and cytochrome C proteins, the functional integrity of individual mitochondria can be characterized.
Instrument configurationThe Flow NanoAnalyzer is equipped with a 488 nm CW laser, and the detection channels are side scatter, green fluorescence (FITC) and red fluorescence (APC).
Results
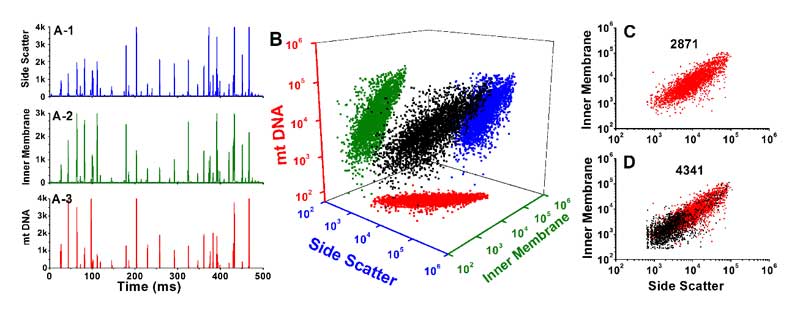
Figure 1. Analysis of mitochondria double-stained with NAO and SYTO 62.
DiscussionBy simultaneous labeling mitochondrial inner membrane protein and mtDNA, Flow NanoAnalyzer demonstrates the assessment of the purity and structural integrity of individual mitochondria via concurrent analysis of side scatter, cardiolipin and mtDNA.
Flow NanoAnalyzer can characterize the functional integrity of individual mitochondria via simultaneous detection of side scatter, porin (outer membrane) and cytochrome C (intermembrane space) proteins essays services reviews.com.
Flow NanoAnalyzer proves a powerful platform for purity and structural integrity estimation, as well as the proteins and nucleic acid detection at the single organelle level. It is expected to become an advanced analytical method for researches on signal transduction of mitochondria-mediated apoptosis pathway.Anal. Chem. 2012, 84, 6421-6428.
2. Detection of ΔΨm and Identification of Mitochondria-Targeting Anticancer Drugs
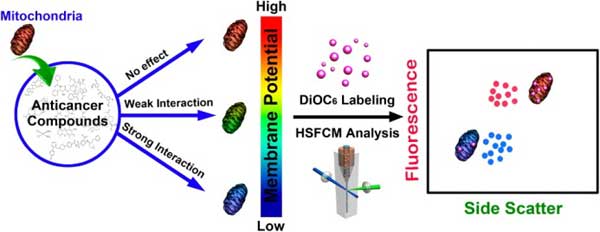
Introduction
Mitochondria play a pivotal role in determining the point-of-no-return of the apoptotic process. Therefore, anticancer drugs that directly target mitochondria hold great
potential to evade resistance mechanisms that have developed toward conventional chemotherapeutics. We report the development of an in vitro strategy to quickly identify the therapeutic agents that induce apoptosis via directly affecting mitochondria. This result is achieved by treating isolated mitochondria with potential anticancer compounds, followed
by simultaneously measuring the side scatter and mitochondrial membrane potential (ΔΨm, labeling with DiOC6(3)) fluorescence of individual mitochondria using Flow NanoAnalyzer. The feasibility of this method was tested with eight widely used anticarcinogens. A potent mitochondrial membrane uncoupling agent, CCCP, was used as positive control.
Instrument configurationThe Flow NanoAnalyzer is equipped with a 488 nm CW laser, and the detection channels are side scatter and green fluorescence (FITC).
Results
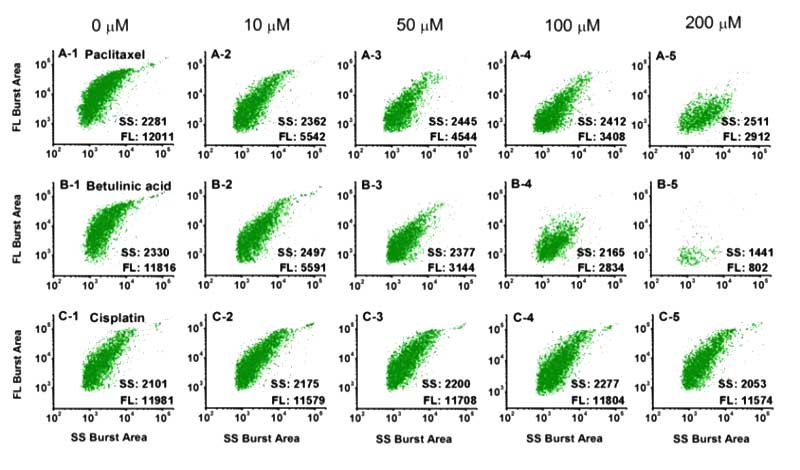
Figure 2. Analysis of mitochondria treated with different anticancer drugs on the Flow NanoAnalyzer.
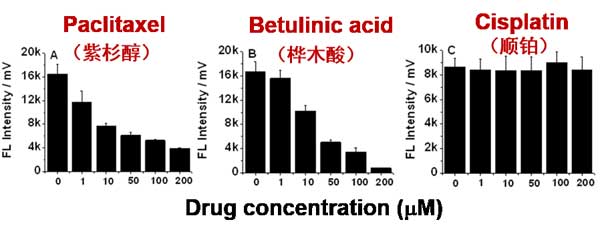
Figure 3. Drug concentration effect on mitochondrial membrane potential of isolated mitochondria.
Discussion
-
By labeling with DiOC6(3), Flow NanoAnalyzer can monitor the change of mitochondrial membrane potential.
-
This method serves an in vitro strategy to quickly identify the therapeutic agents that induce apoptosis via directly affecting mitochondria, and side scatter detection can reveal the change of internal contents upon drug treatment at the single-organelle level with high resolution.
-
Flow NanoAnalyzer provides an advanced analytical method for anticancer mechanism studies and drug screening.
Anal. Chem. 2014, 86, 5232-5237.
3. Quantification of Protein Copy Number in Single Mitochondria
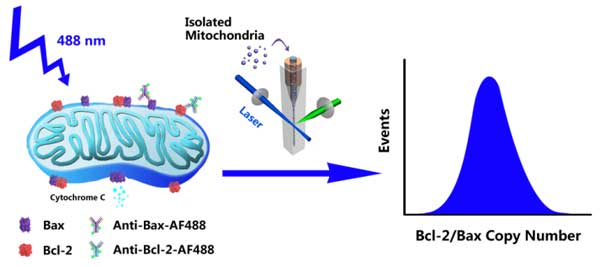
IntroductionBcl-2 family proteins, represented by antiapoptotic protein Bcl-2 and proapoptotic protein Bax, are key regulators of mitochondria-mediated apoptosis pathway. To build a quantitative model of how Bcl-2 family protein interactions control mitochondrial outer membrane permeabilization and subsequent cytochrome C release, it is essential to know the number of proteins in individual mitochondria. Here, we report an effective method to quantify the copy number and distribution of proteins in single mitochondria via immunofluorescent labeling and sensitive detection by Flow NanoAnalyzer.
Instrument configurationThe Flow NanoAnalyzer is equipped with a 488 nm CW laser, and the detection channels are side scatter, green fluorescence (FITC) and red fluorescence (APC).
Results
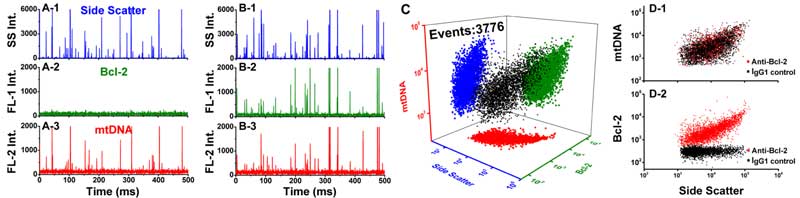
Figure 4. Analysis of mitochondria double-stained with AF488-labeled mAb and SYTO 62.
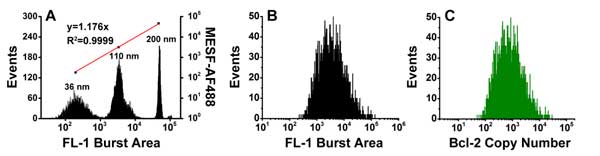 Figure 5. Quantitative measurement of Bcl-2 copy number in individual mitochondria.
Figure 5. Quantitative measurement of Bcl-2 copy number in individual mitochondria.
Discussion
-
Membrane-permeable nucleic acid dye SYTO 62 was used to stain mtDNA for the identification of intact mitochondria.
-
A series of fluorescent nanospheres with fluorescence intensity calibrated in the unit of molecules of equivalent soluble fluorochrome (MESF)-AF488 were used to construct a calibration curve for converting the immunofluorescence of a single mitochondrion to the number of antibodies bound to it and then to the number of proteins per mitochondrion.