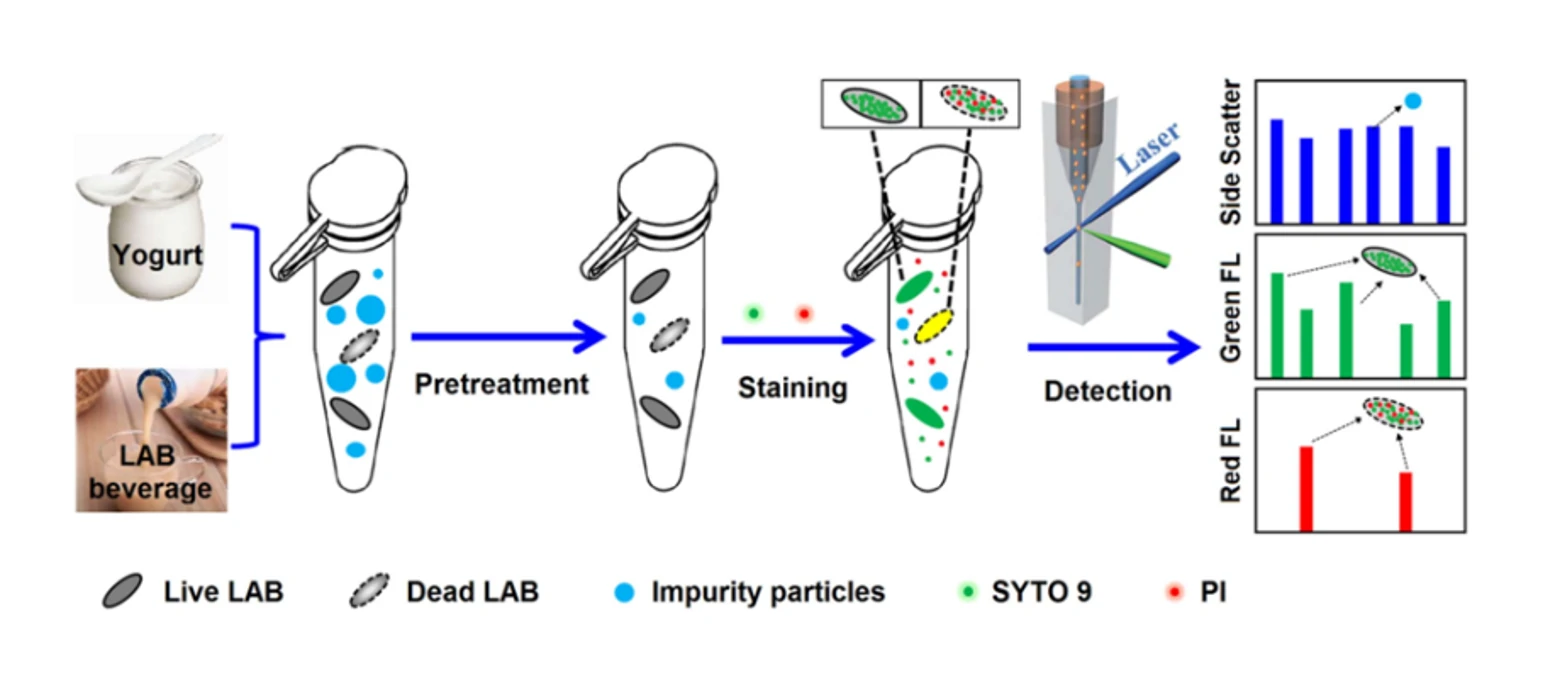
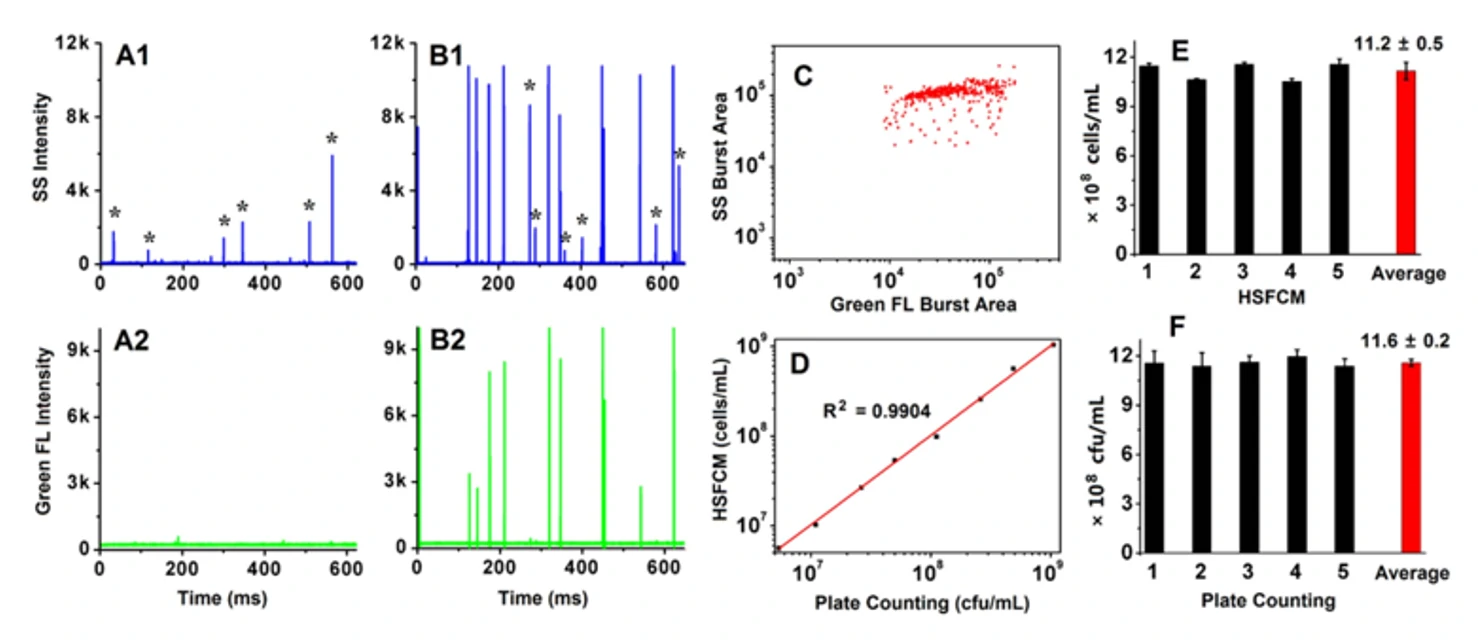
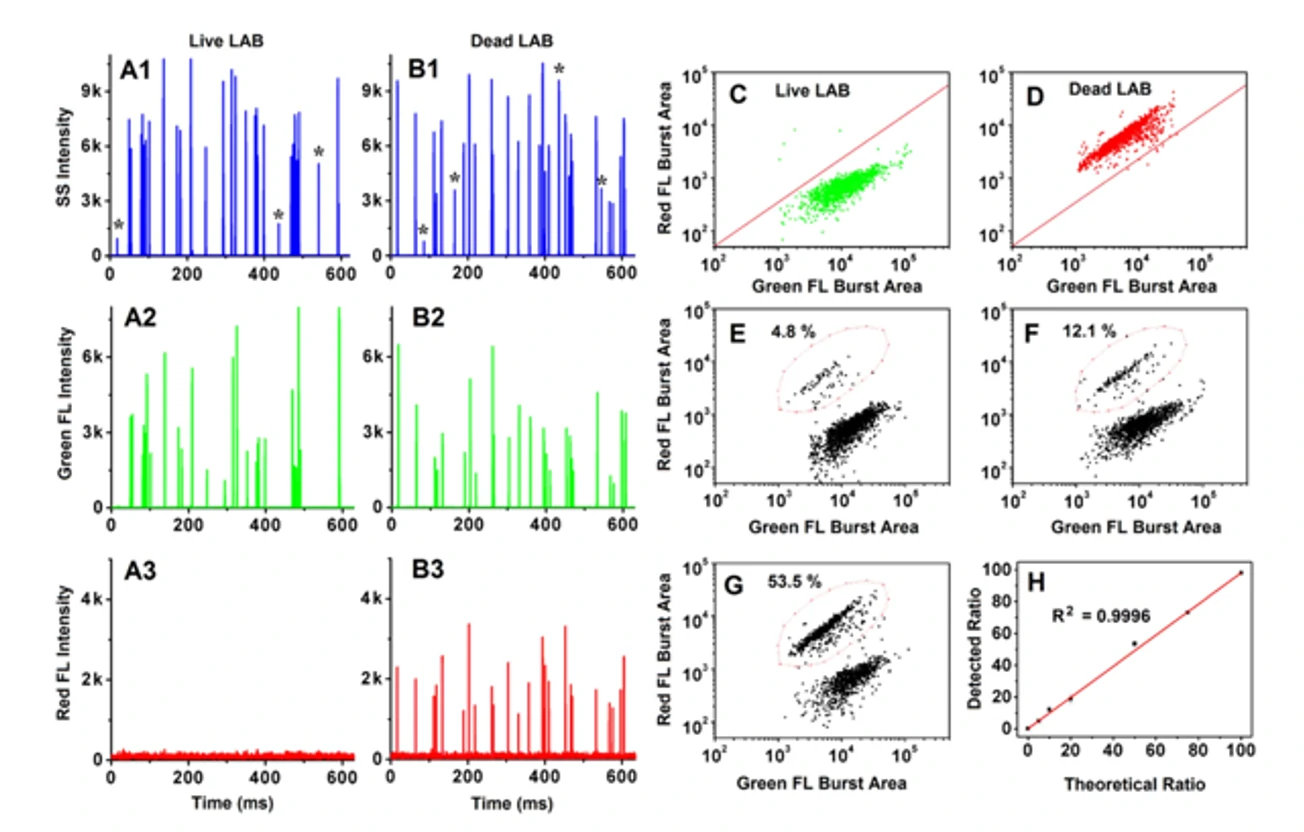
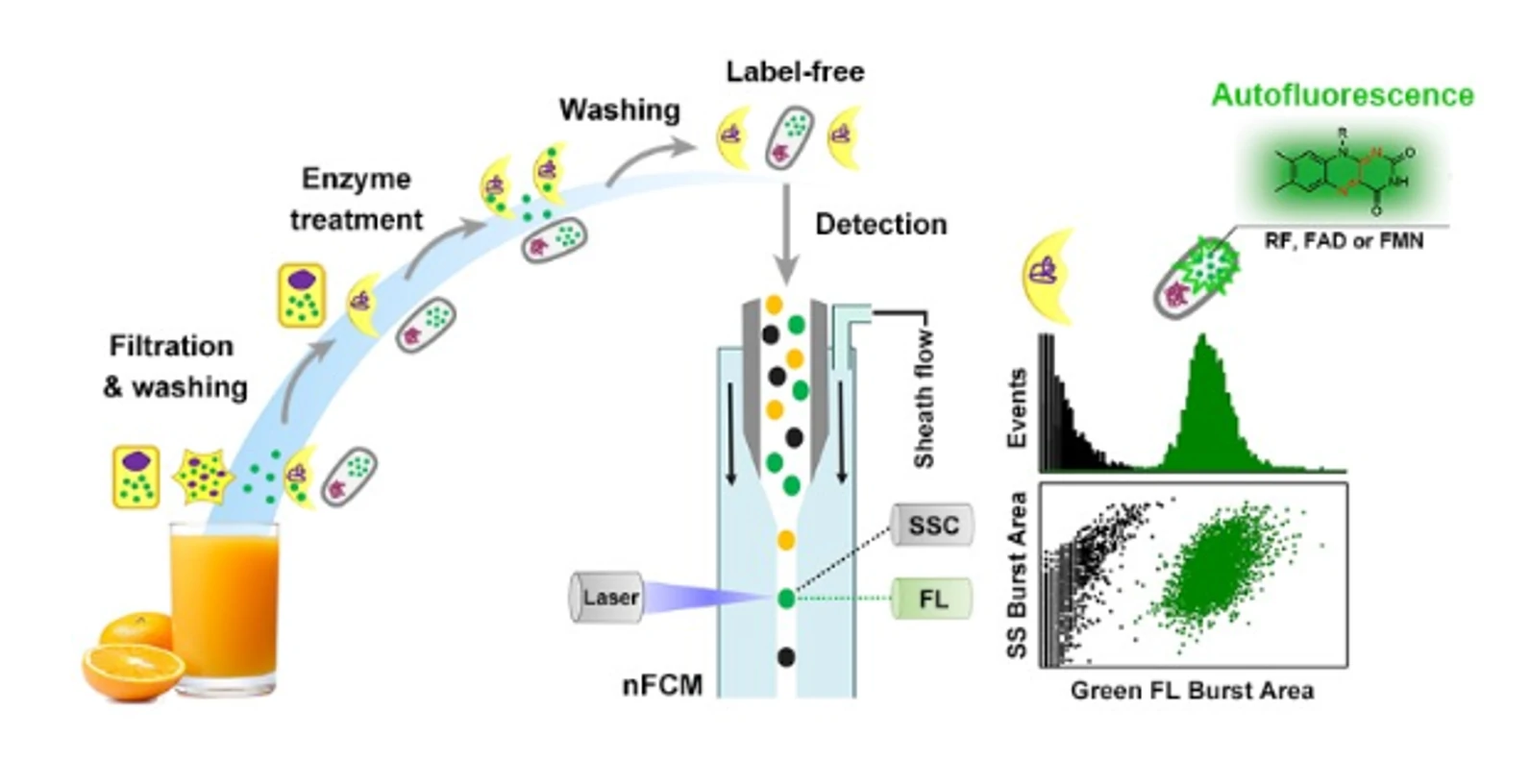
Quantification of Total Bacterial Cells in Water and Drink
A safe and secure supply of drinking water is an essential requirement for human health. Due to the potential occurrence of various microbiological contaminants in drinking water and beverages, the total bacterial count represents a key parameter for quality assessment. Currently, water dispensers are commonly used, and the fluctuation of water quality, especially the bacterial count of unsealed barreled water, has attracted much attention. The cultivation-based heterotrophic plate count (HPC) has long been a firmly established tool for the assessment of water quality. However, it is labor-intensive, time-consuming, and of limited use in certain circumstances. On the other hand, bacteria in a state of very low metabolic activity, such as viable but non-cultivable (VBNC) bacteria, can be overlooked by HPC.
By utilizing the nucleic acid dye PicoGreen to label particles in water, particles that show burst traces in both side scatter and fluorescent channels simultaneously are recognized as bacteria. Compared with HPC, the Flow NanoAnalyzer-based approach not only shortens the analysis time but also reveals the presence of dead and VBNC bacterial cells.
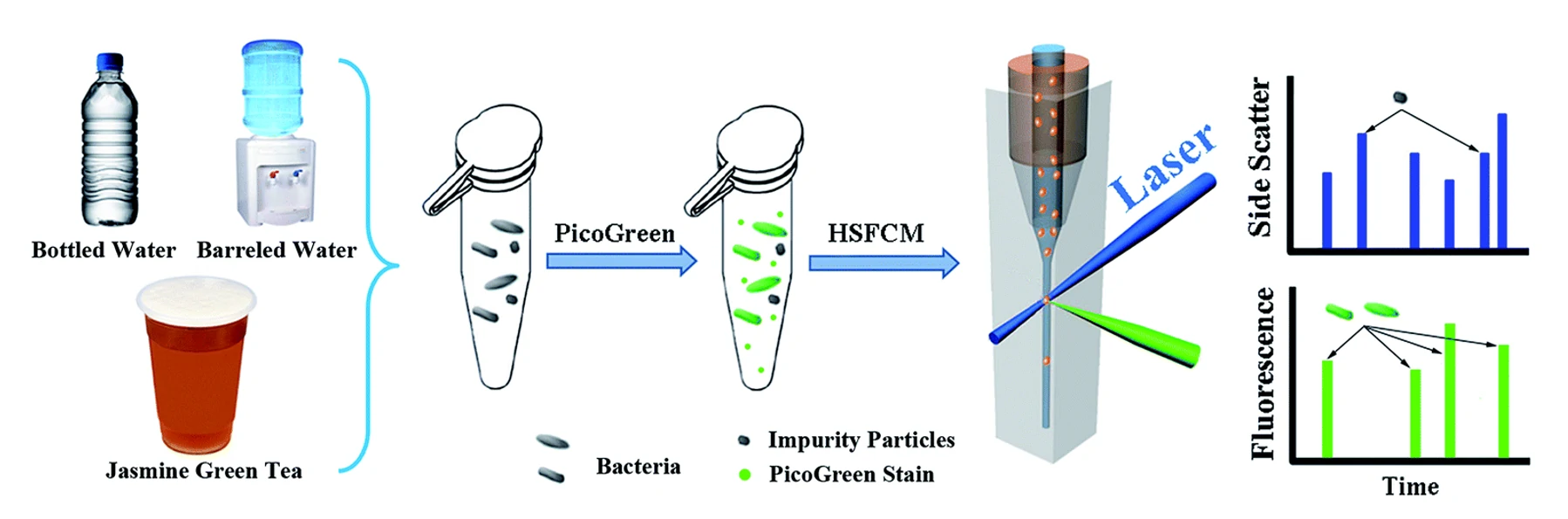
Combined with PicoGreen nucleic acid fluorescence staining, rapid and accurate quantification of total bacteria in drinking water and tea beverages is achieved, and the results correlates well with conventional HPC method.
Anal. Methods, 2015, 7(7), 3072-3079.