Colorectal Cancer
EVs are associated to many pathological conditions such as thrombosis, haemostasis, inflammation, sickle cell disease and malaria. They may serve as biomarkers of disease and therapeutic targets. A deeper understanding of the cargo molecules present in EVs obtained from patients with cancers will aid in the identification of novel diagnostic and prognostic biomarkers, and could potentially lead to the discovery of new therapeutic targets. The detection of biomarkers in body fluids has major advantages over the use of tissue markers, which often require invasive biopsies that can be difficult to perform and potentially dangerous.
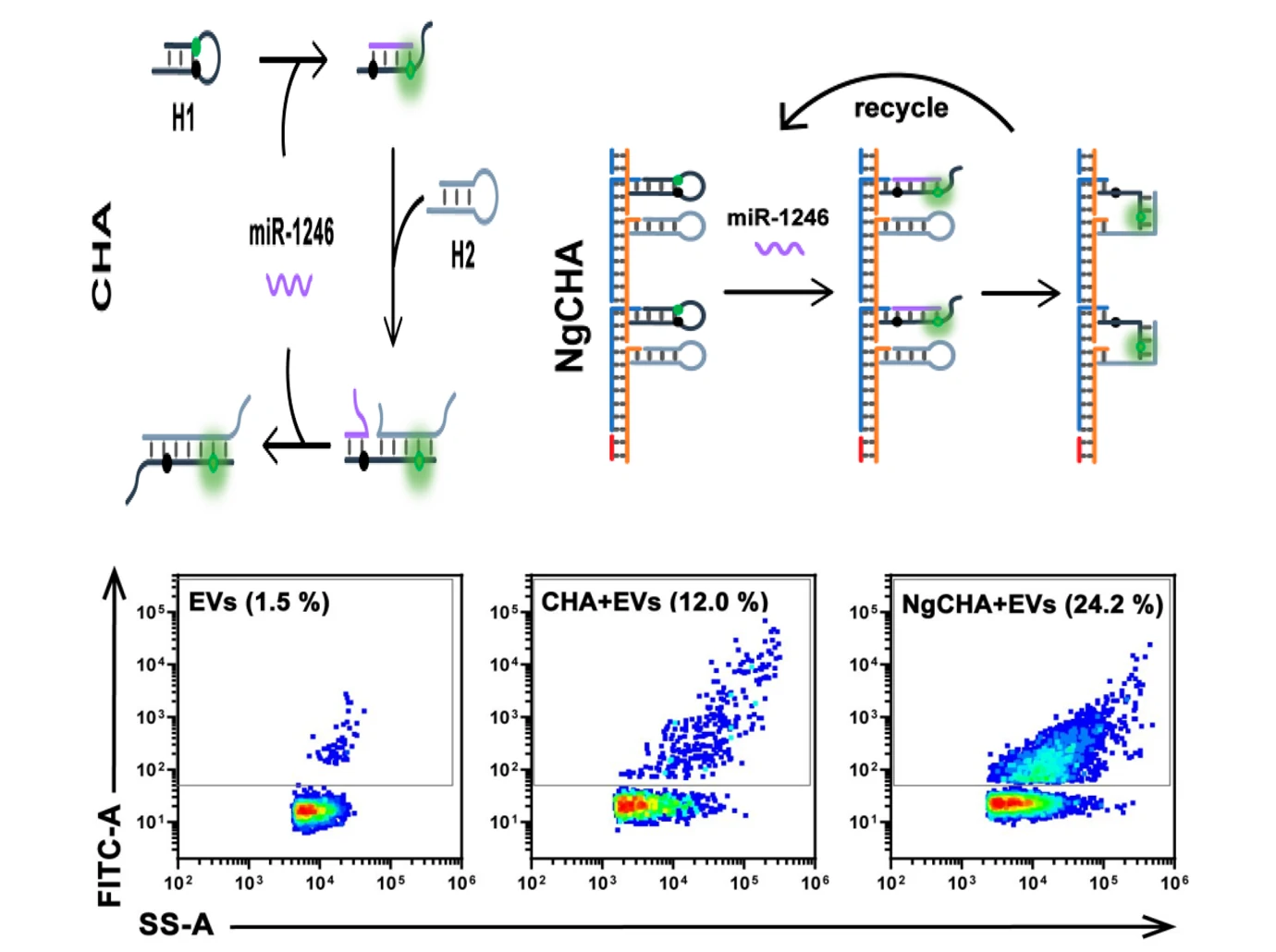
Although various methods exist to quantify EVs, EV quantification in clinical samples remains challenging and more importantly, current approaches are often unable to identify EV subpopulations. Here, we provide a nano-flow cytometry based assay for the early diagnosis of colorectal cancer.
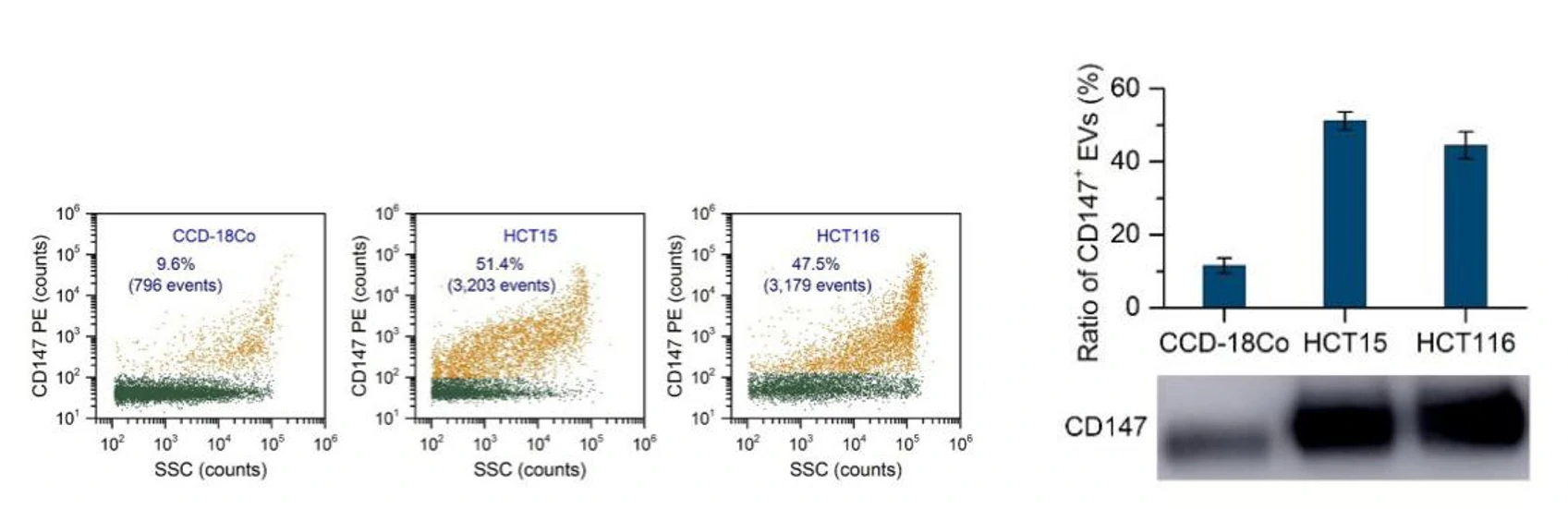
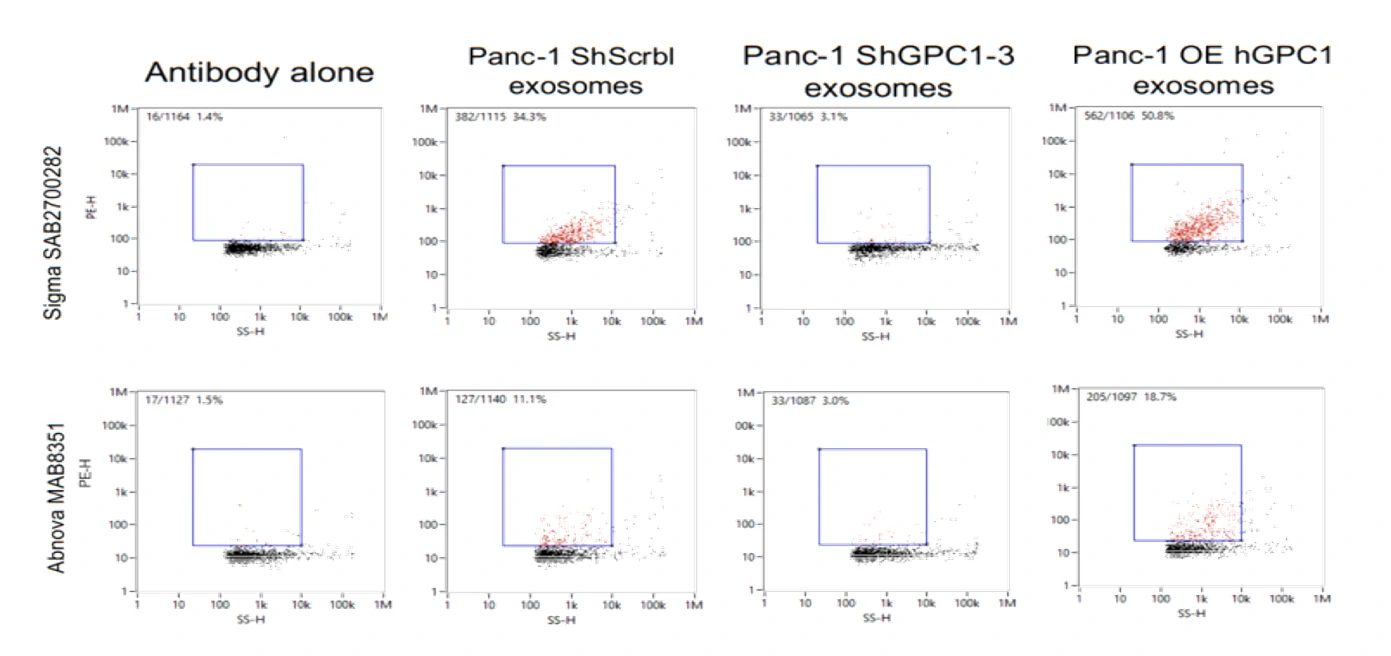
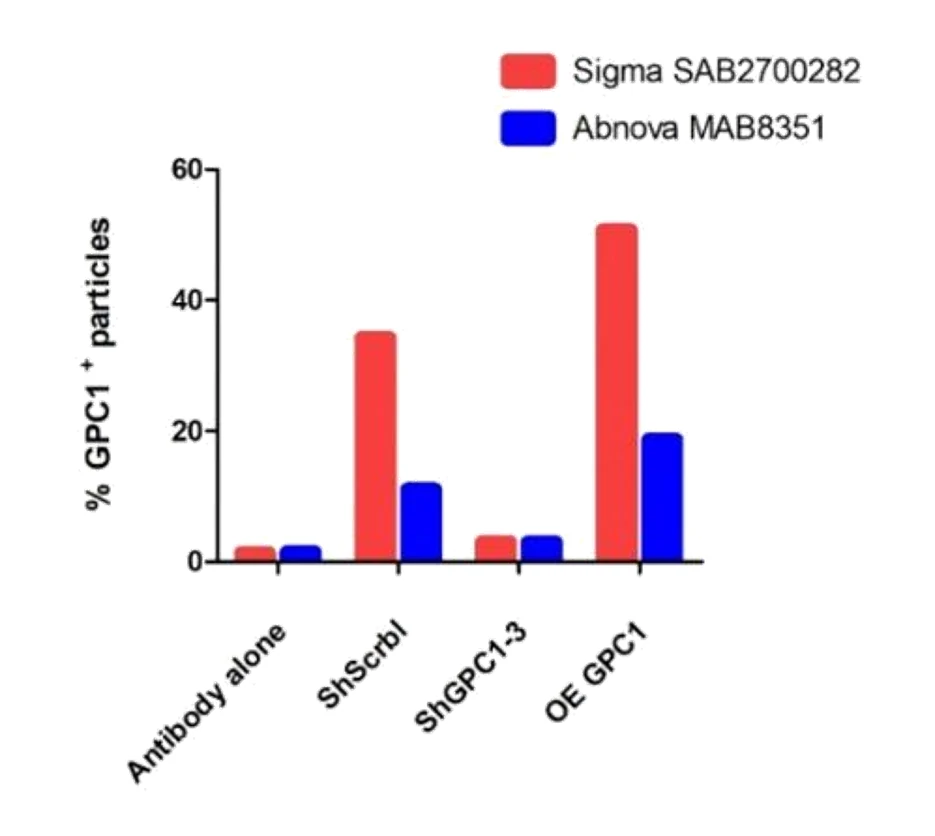
Figure 1. Protein profiling of single EVs isolated from cell culture. Figure 2. Western blotting analysis of CD147-positive EVs.
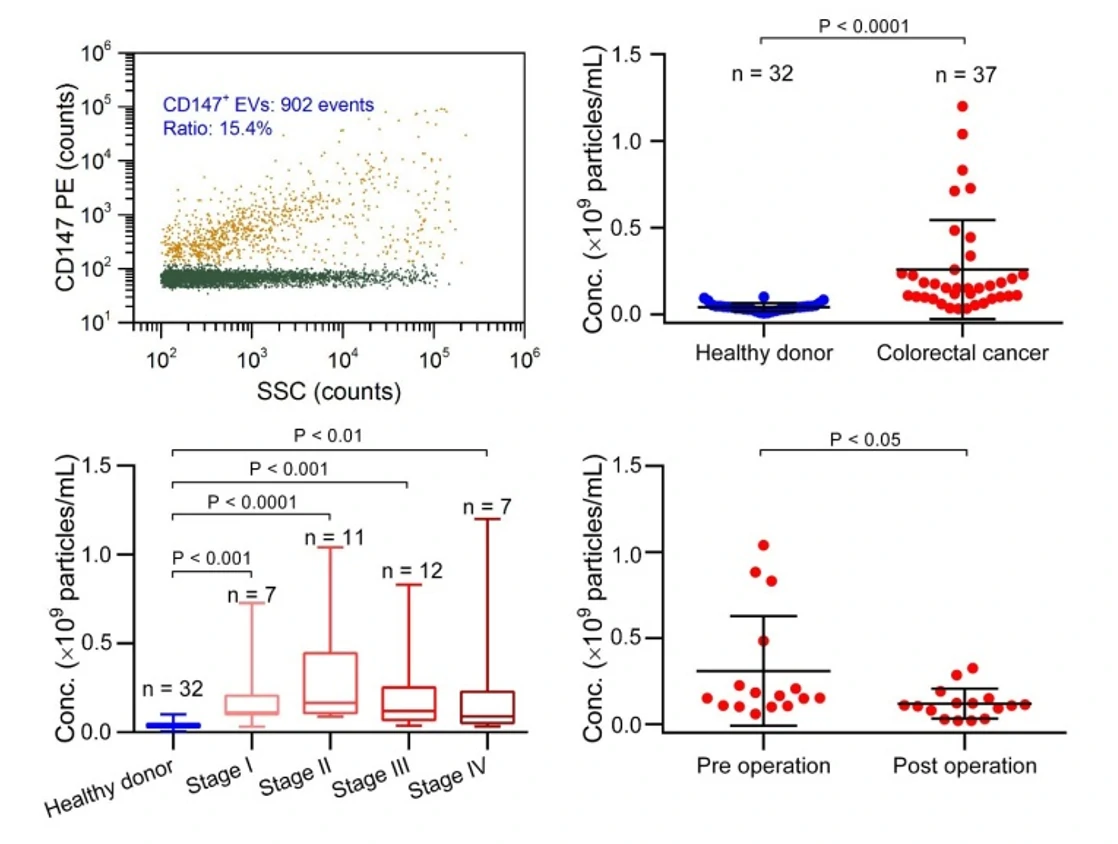
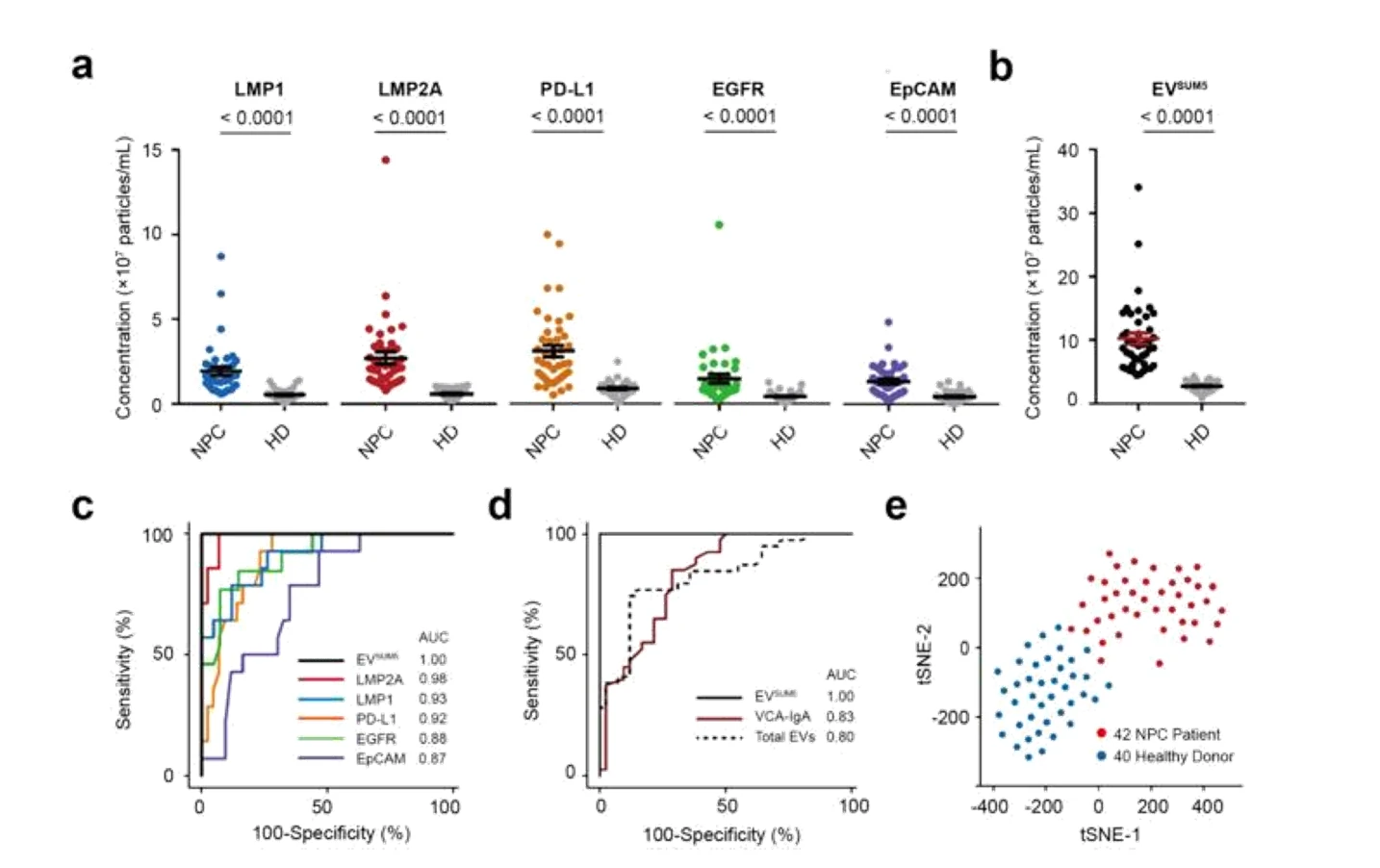
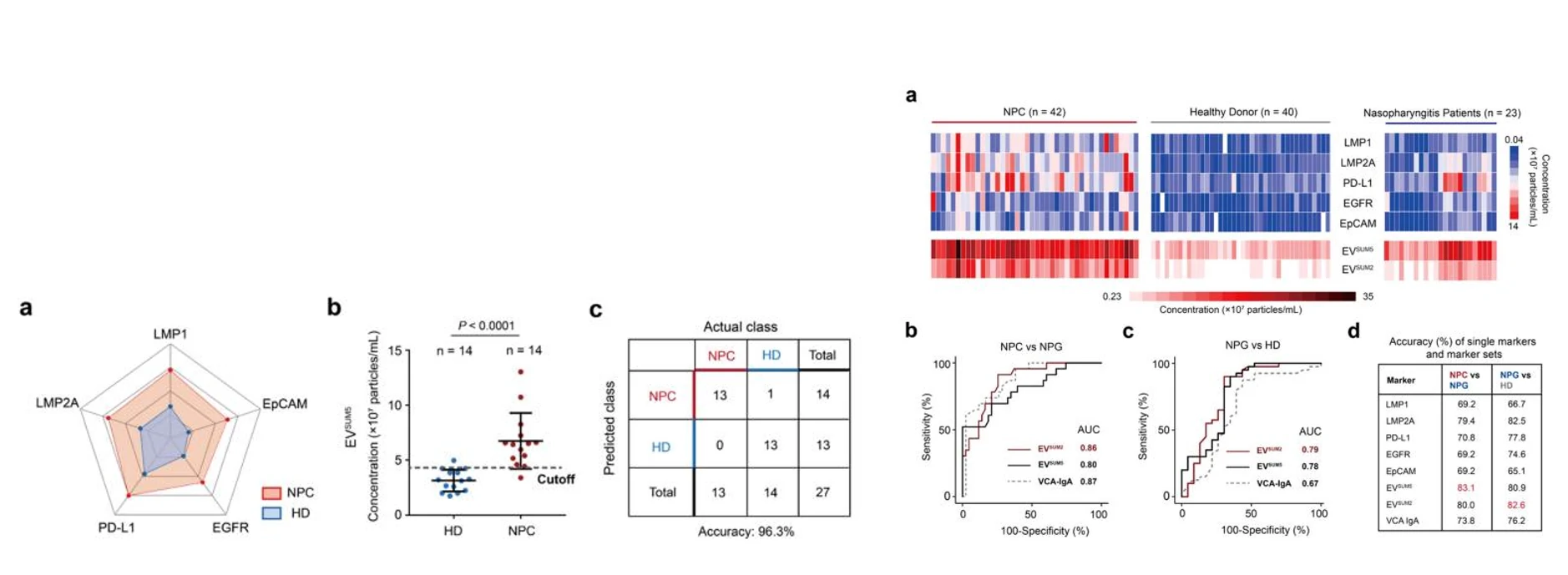
Figure 3. EVs for the early diagnosis of colorectal cancer.
The Flow NanoAnalyzer enables discrimination of EVs extracted from plasma of colorectal cancer patients and healthy donors after immunofluorescent labeling.
ACS Nano, 2018, 12(1), 671-680.