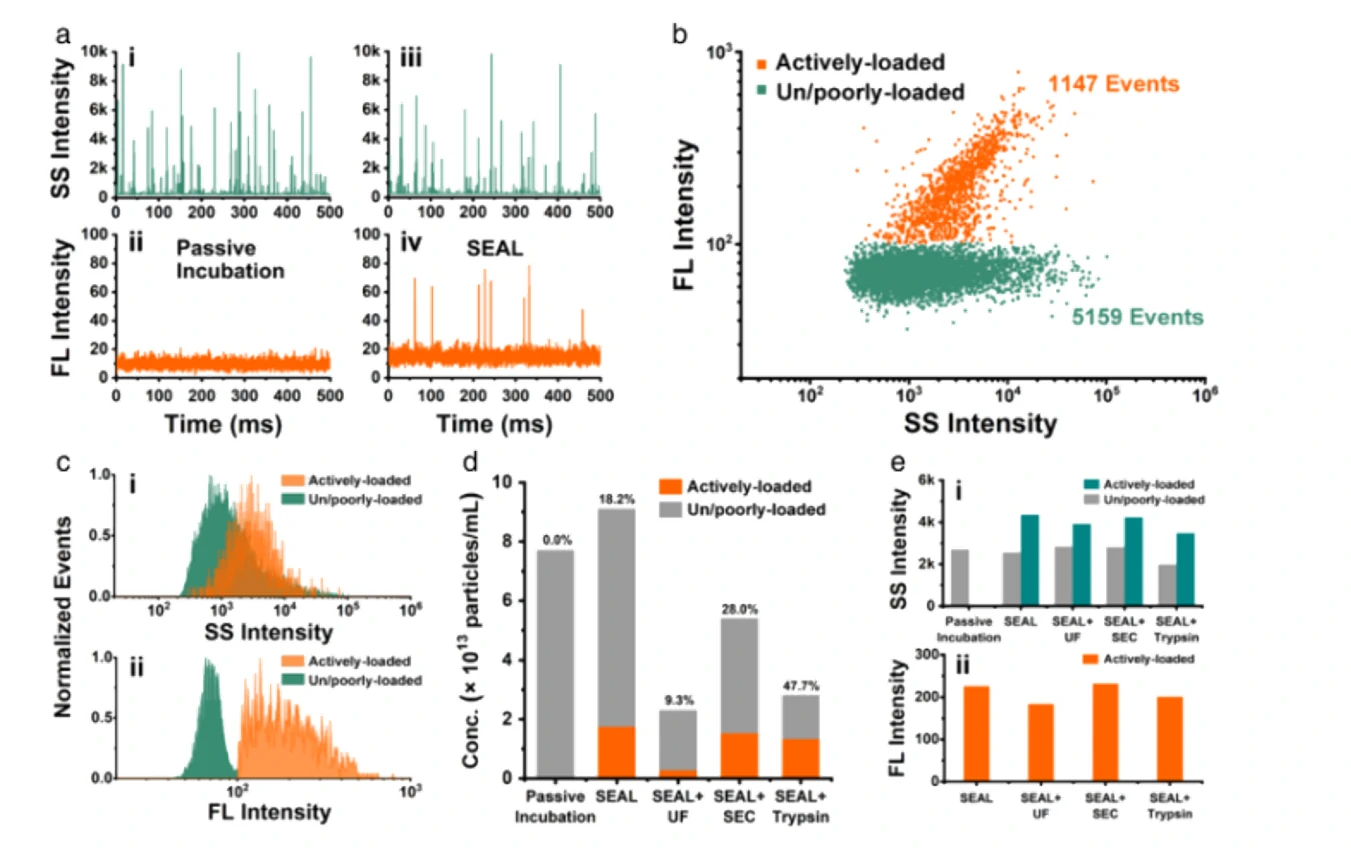
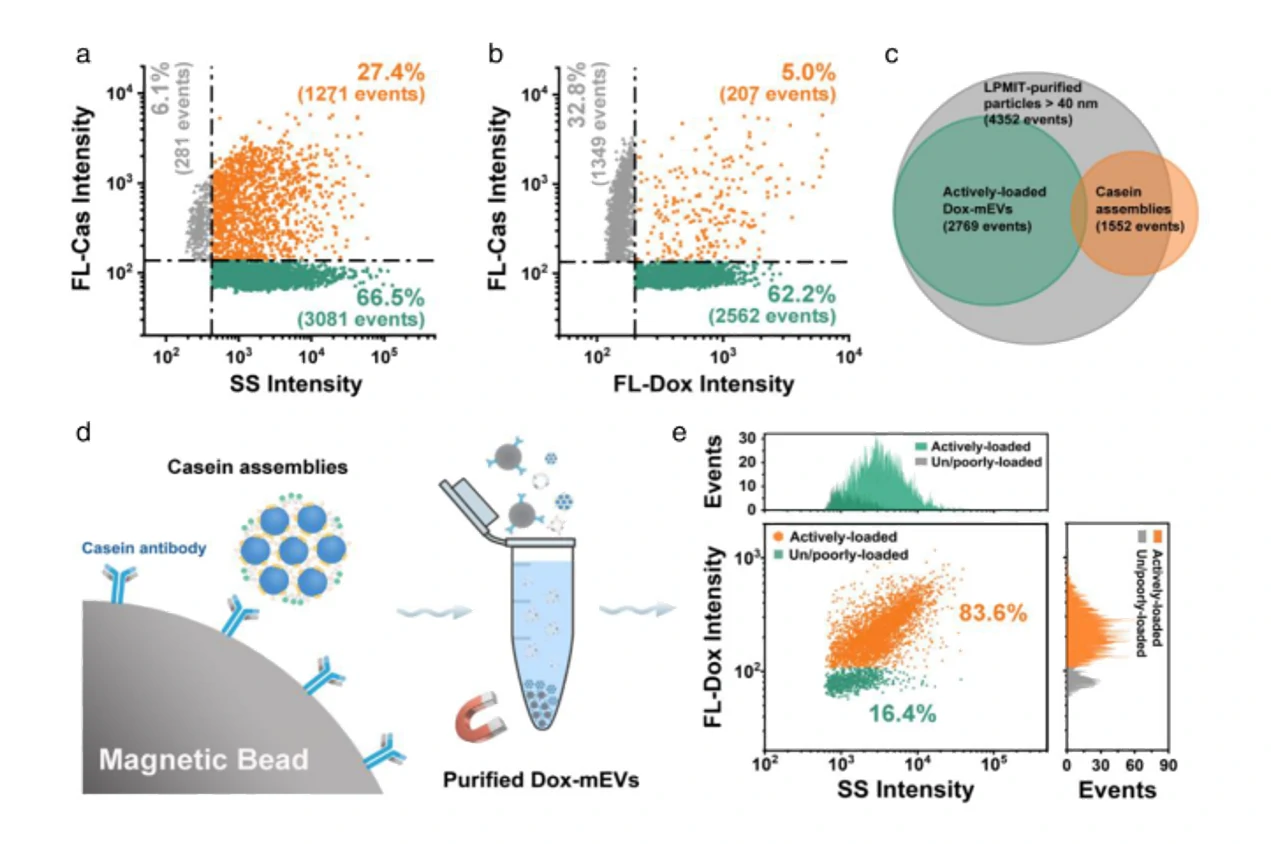
Six different drug-loading strategies
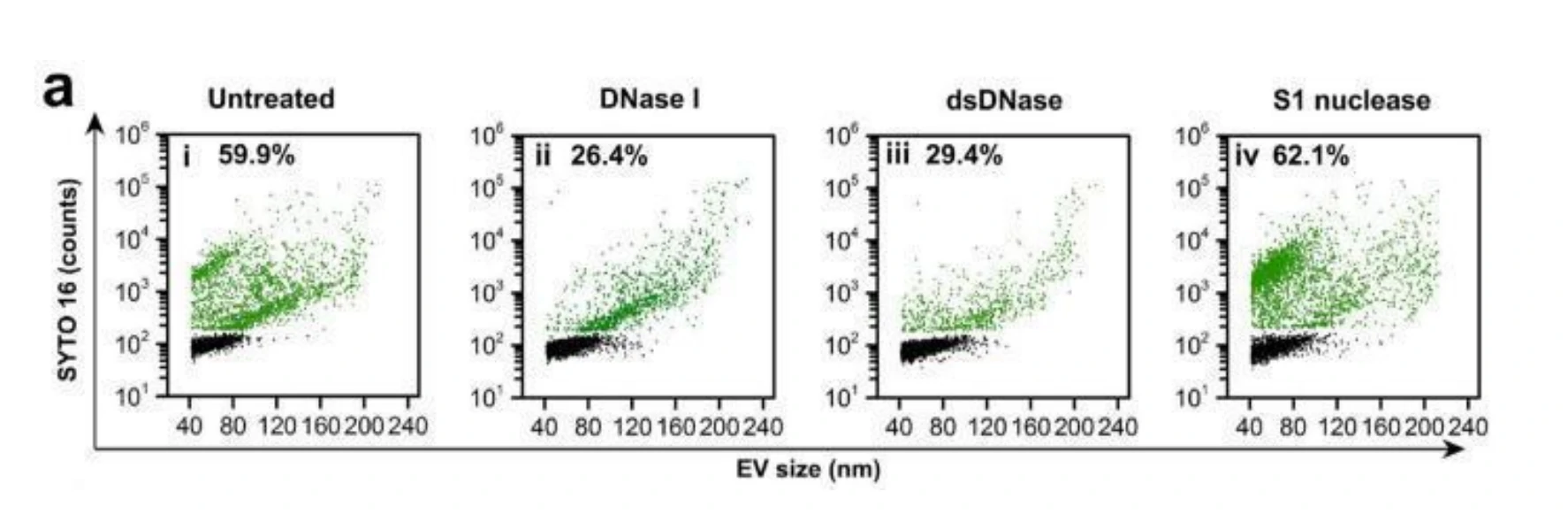
Extracellular vesicles (EVs) have emerged as an attractive drug delivery system owing to their natural roles in intercellular communication. Due to large intrinsic heterogeneity of EVs, it is highly desirable to evaluate not only the encapsulation efficiency but also the alteration of biological functionality after the drug-loading process at the single-particle level. However, the small size of EVs poses a great challenge. Taking advantage of the Flow NanoAnalyzer multiparameter analysis of single EVs as small as 40 nm, six commonly used drug-loading strategies (coincubation, electroporation, extrusion, freeze-thawing, sonication, and surfactant treatment) were explored by employing doxorubicin (Dox) as the model drug. Encapsulation ratio, EV concentration, drug content, and membrane proteins of Dox-loaded EVs were measured at the single-particle level. Data indicated that coincubation and electroporation outperformed other methods with an encapsulation ratio of approximately 45% and a higher Dox content in single EVs. Interestingly, the labeling ratios of membrane proteins indicated that varying degrees of damage to the surface proteins of EVs occurred upon extrusion, freeze-thawing, sonication, and surfactant treatment.
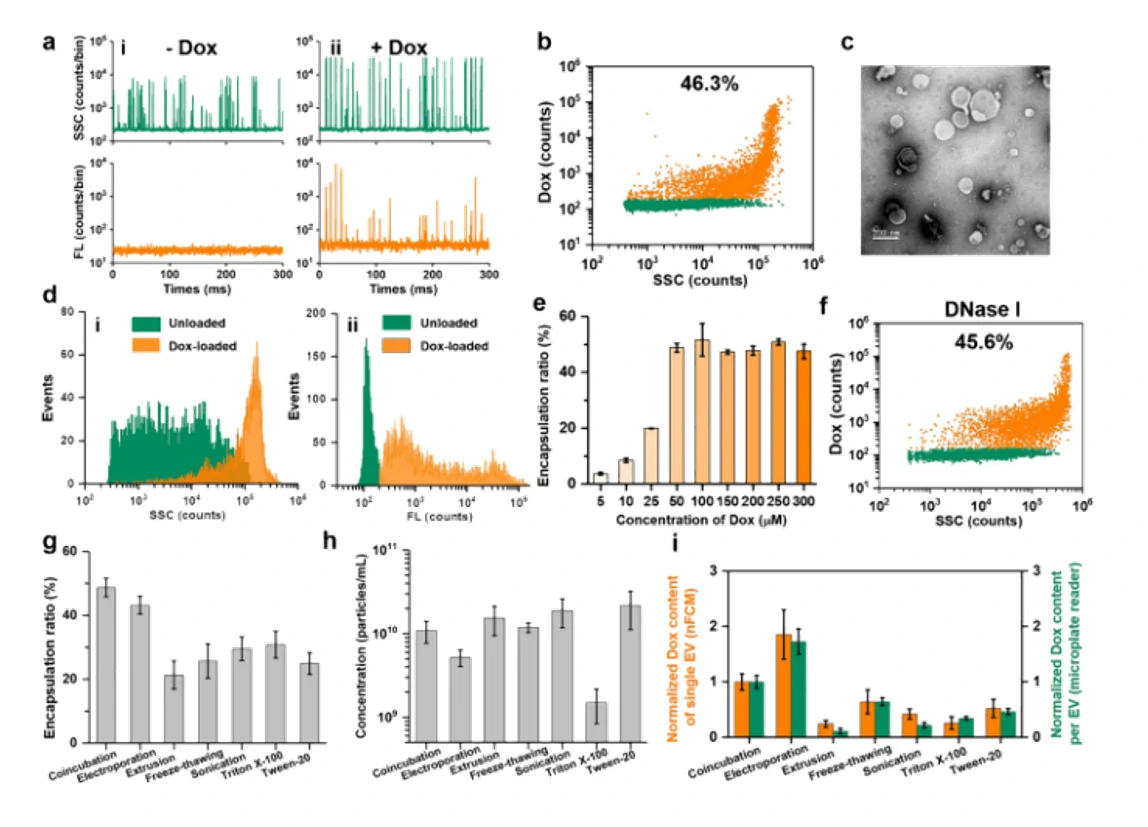
Figure 1. Encapsulation ratio of Dox-loaded EVs in different drug-loading strategies
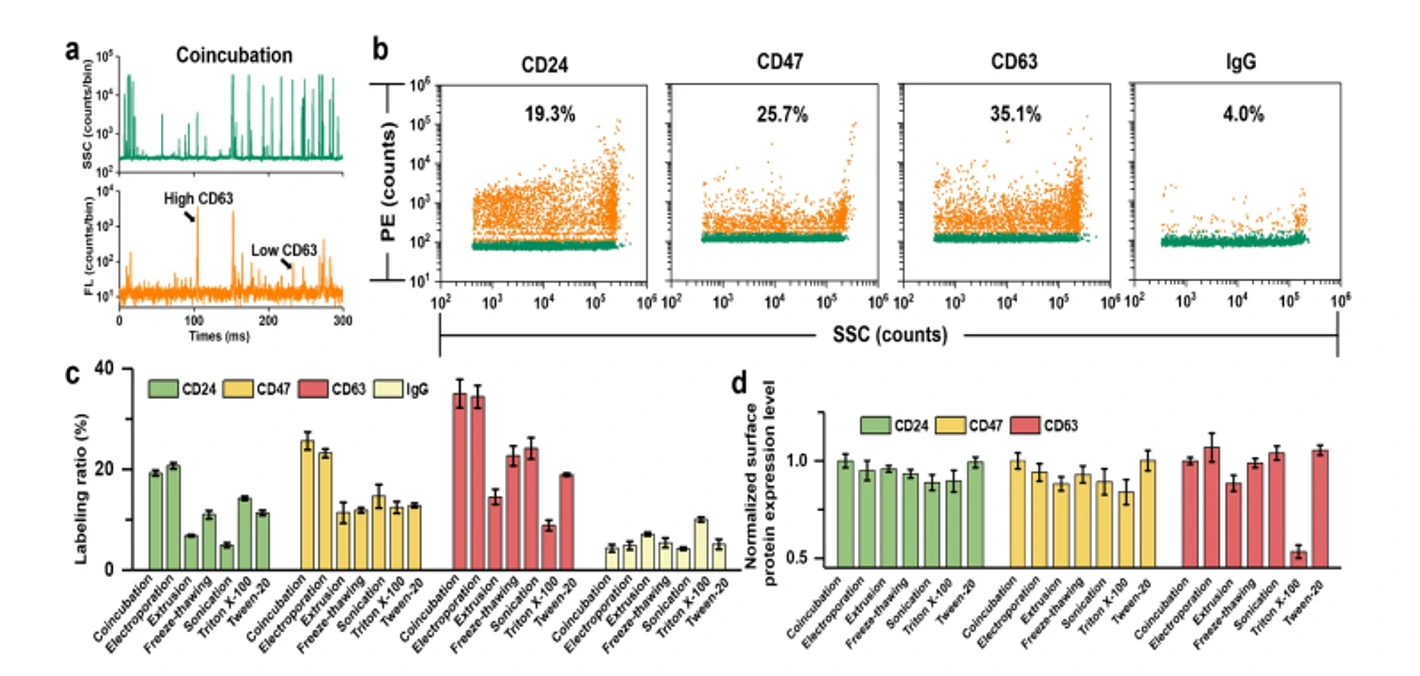
Figure 2. The expression of membrane proteins of Dox-loaded EVs in differemt drug-loading strategies
Confocal fluorescence microscopy and nano-flow cytometry analysis revealed that Dox-loaded EVs prepared by electroporation induced the strongest apoptosis followed by coincubation. These results correlated well with their cellular uptake rate and fundamentally with the Dox encapsulation efficiency of single EVs. Flow The NanoAnalyzer provides a rapid and sensitive platform for single-particle assessment of drug-loading strategies for incorporating drugs into EVs.
Anal Bioanal Chem., 2022. https://doi.org/10.1007/s00216-022-04248-4.