Comparison of Commercial Isolation kits
Extracellular vesicles (EVs) are nanosized particles with significantly varied sizes. In addition, EVs are also heterogeneous in composition, and enriched in membrane-associated, high-order oligomeric protein complexes. EVs are being developed as diagnostic and therapeutic agents in multiple disease models. Isolation of EVs from complex biological fluids with high purity is essential to the accurate analysis of EV cargo. Unfortunately, commonly used isolation techniques do not offer good separation of EVs from non-EV contaminants. Hence, it is important to have a standardized method to characterize the properties of EV preparations, including size distribution, particle concentration, purity and phenotype. The current common methods of exosomes purification include; differential ultracentrifugation, size exclusion, exosome precipitation and immunoaffinity. Characterization of EVs single particle level is of great importance to investigate the mechanism of vesicle transport systems, cell communication, early diagnosis, and disease treatment. The current techniques for single particle detection include cryoTEM, nanoparticle tracking analysis (NTA), tunable resistive pulse sensing (TRPS). However, with exception to cryoTEM, the other techniques struggle to detect particles smaller than 70 nm and only provide size distribution and particle concentration.
The Flow NanoAnalyzer enables multiparameter analysis of single EVs as small as 40 nm, a new benchmark for the quality and efficiency assessment of EVs isolated from plasma is reported. Properties, such as size distribution, particle concentration, purity, recovery rate and surface proteins, were measured. The performance of five widely used commercial isolation kits was examined and compared with the commonly used technique of differential ultracentrifugation.

Figure 1. The particle size distribution and concentration Figure 2. Particle concentration of
of EVs preparation from PFP by different methods. EV preparations from PFP and VD-PFP.

Figure 3. Measurement of the purity of different Figure 4. Measurement of the recovery rates of
isolation methods for pure EVs. different isolation methods for pure EVs.

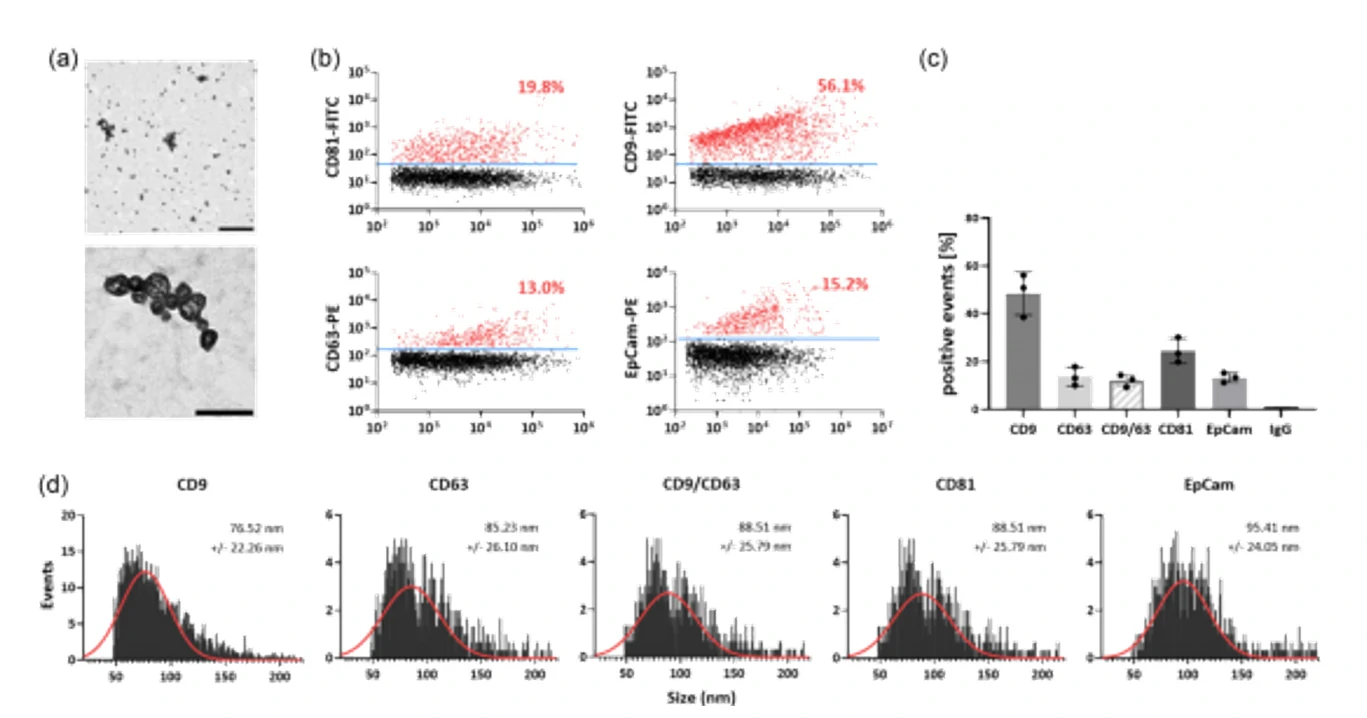
Figure 5. Single-particle phenotyping of EVs isolated by UC from CCCM of HCT15 cells and PFP.
The purity of EVs isolated from PFP by UC was 78.2% in this study, which is only slightly lower than the 90% for EVs isolated from conditioned cell culture medium. In contrast to the much higher yields of commercial isolation kits, the measured purity (ranging from 28.1 to 5.3%) was markedly lower in comparison to UC.
To compare characterization of surface proteins on EVs, the EVs extracted by the kit also need to be further removed by the ultra-ionization method to obtain results consistent with UC. In conclusion, there is a significant difference between commonly used isolation kits and traditional ultracentrifugation methods.
J Extracell Vesicles, 2019, 9(1), 1697028.