Mesenchymal stem cell-derived EVs attenuate cartilage injury
The main strategy of tissue repair and regeneration focuses on the application of mesenchymal stem cells (MSCs) and cell-based nanoparticles, but there are still multiple challenges that may have negative impacts on human safety and therapeutic efficacy. Cell-free nanotechnology can effectively overcome these obstacles and limitations. MSC-derived natural small extracellular vesicles (sEVs) represent ideal nanotherapeutics due to their low immunogenicity and lack of tumorigenicity. Here, sEVs harvested from Whartons jelly mesenchymal stem cells (WJMSCs) were identified. In vitro results showed that WJMSC-sEVs efficiently entered chondrocytes in the osteoarthritis (OA) model, promoting chondrocyte migration and proliferation and modulating immune reactivity. In vivo, WJMSC-sEVs notably promoted chondrogenesis, which was consistent with the effect of WJMSCs. RNA sequencing results revealed that sEV-microRNA-regulated biocircuits can significantly contribute to the treatment of OA, such as by promoting the activation of the calcium signaling pathway, ECM-receptor interaction pathway and NOTCH signaling pathway.
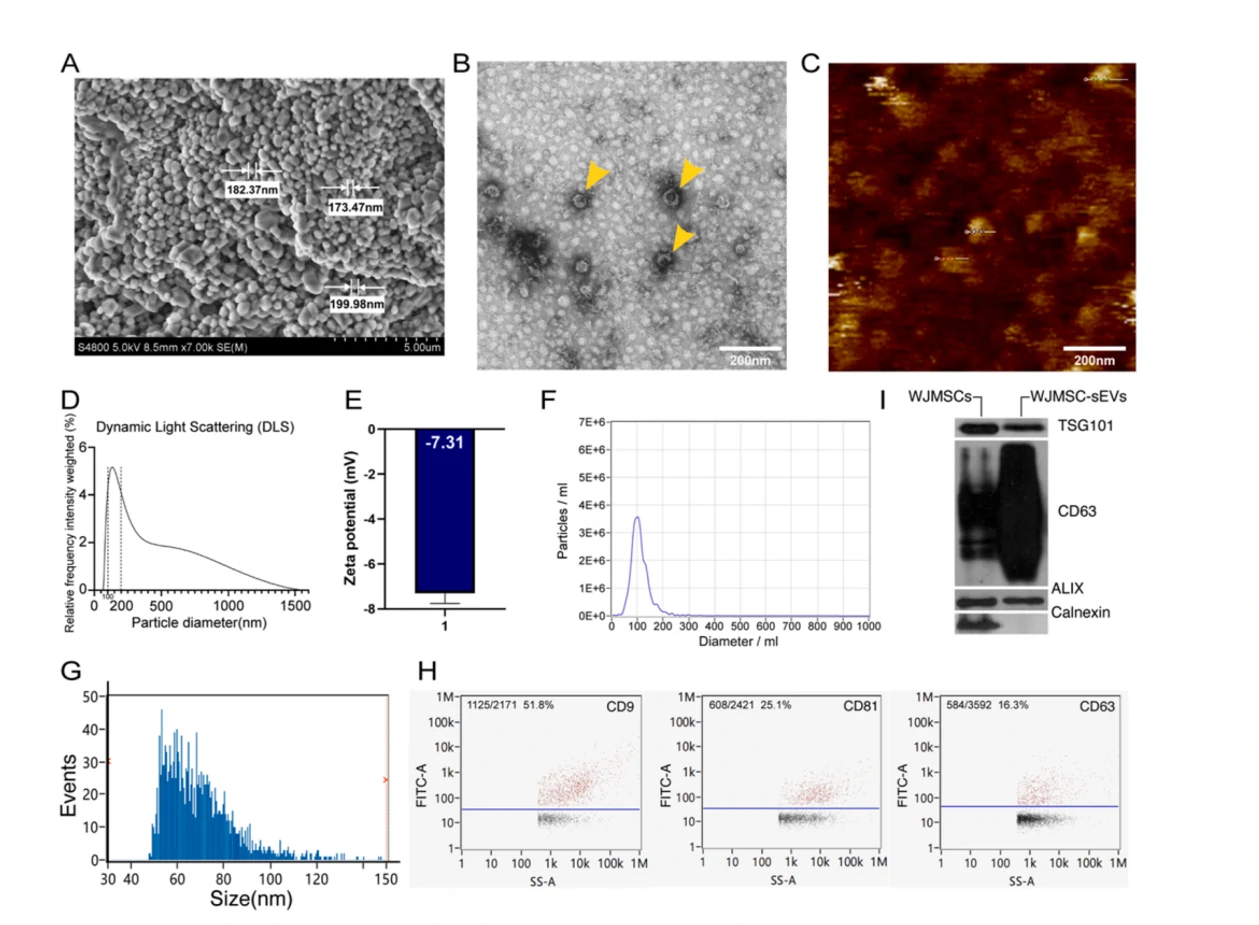
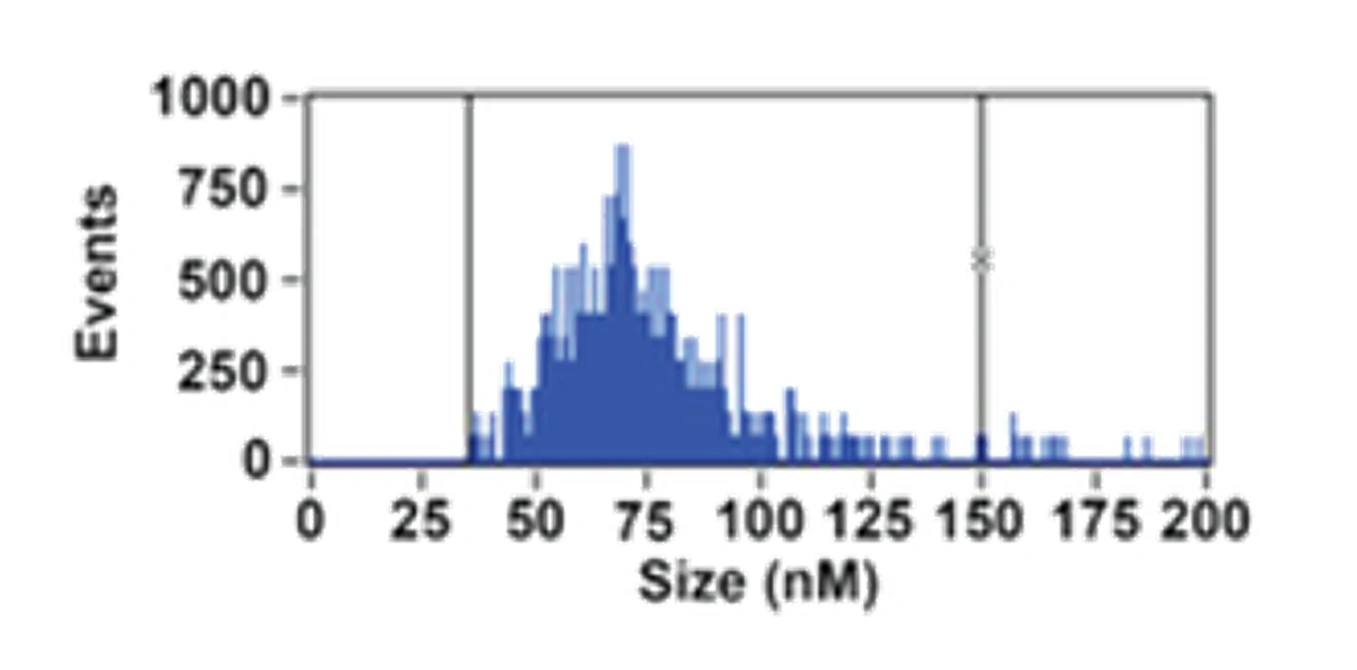
Figure 1. Comprehensive characterization of WJMSCs-sEVs
The Flow NanoAnalyzer characterizes sEV size distribution, particle concentration and surface proteins at the single particle level, laying the foundation for the application of stem cell-derived exosomes for tissue repair and regeneration.
Int J Pharm., 2022, 623:121952.