Estimation of Purity and Structural Integrity of Single Mitochondria
Mitochondria play a central role in the regulation of energy metabolism and signal transduction in eukaryotic cells. Mitochondrial dynamics actively contribute to various cellular activities, such as mitophagy, apoptosis, and natural immunity. Mitochondrial dysfunction may lead to several human diseases, including neurodegenerative diseases and metabolic disturbances. Therefore, the study of mitochondria is of great significance for the development of basic life science. However, conventional flow cytometric analysis of individual mitochondria has been limited to attributes that utilize fluorescence staining.
Here, the Flow NanoAnalyzer is applied for the high-throughput multiparameter analysis of individual mitochondria. By simultaneous labeling of mitochondrial cardiolipin (inner membrane) and mtDNA (matrix) with NAO and SYTO 62, respectively, the Flow NanoAnalyzer demonstrates the assessment of the purity and structural integrity of individual mitochondria. By simultaneous labeling of porin and cytochrome C proteins, the functional integrity of individual mitochondria can be characterized.
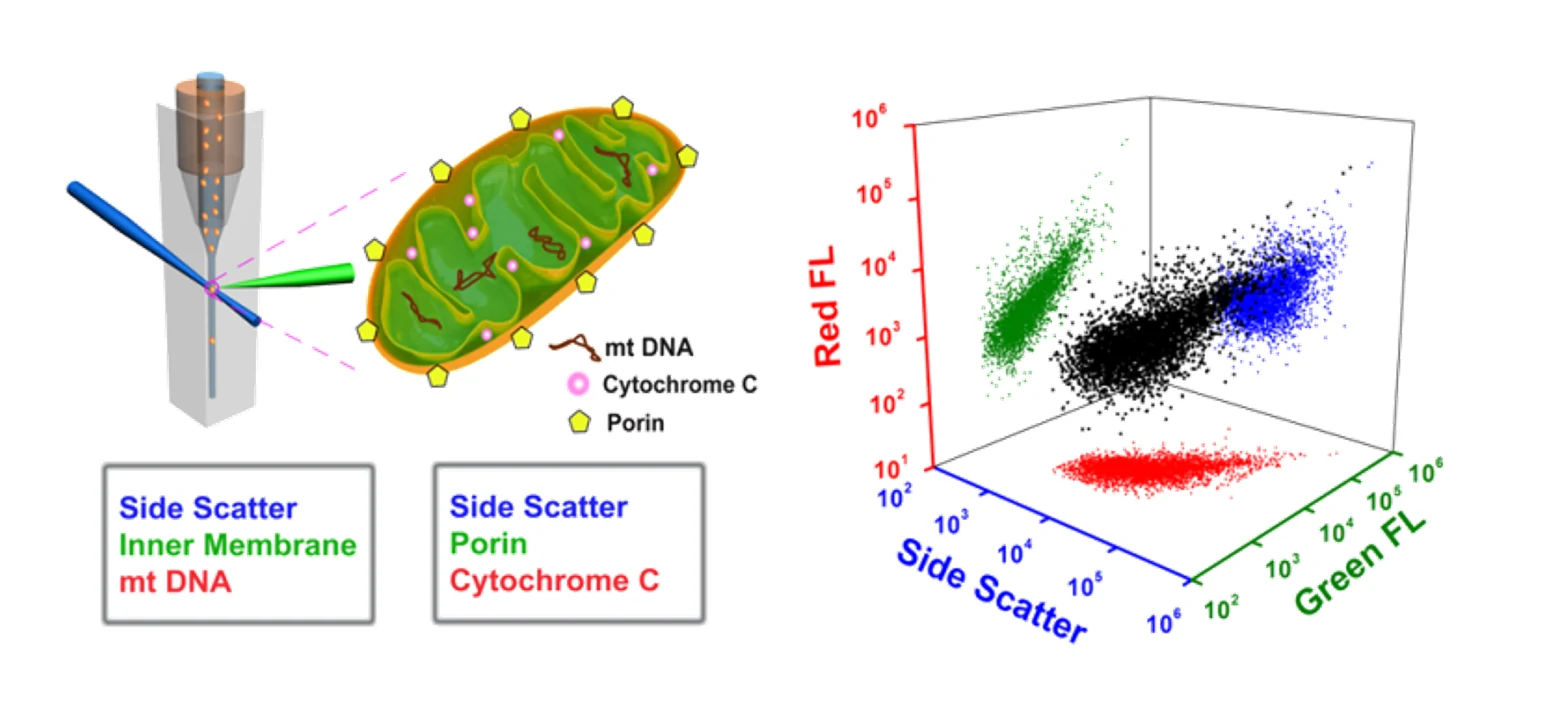
The Flow NanoAnalyzer can characterize the functional integrity of individual mitochondria via simultaneous detection of side scatter, porin (outer membrane) and cytochrome C (intermembrane space) proteins.
Anal. Chem., 2012, 84(15), 6421-6428.
Quantification of Protein Copy Number in Single Mitochondria
Bcl-2 family proteins, represented by the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax, are key regulators of the mitochondria-mediated apoptosis pathway. To build a quantitative model of how Bcl-2 family protein interactions control mitochondrial outer membrane permeabilization and subsequent cytochrome C release, it is essential to know the number of proteins in individual mitochondria. Employing an immunofluorescent labelling strategy, the copy number and distribution of proteins in single mitochondria are accessed via the Flow NanoAnalyzer.
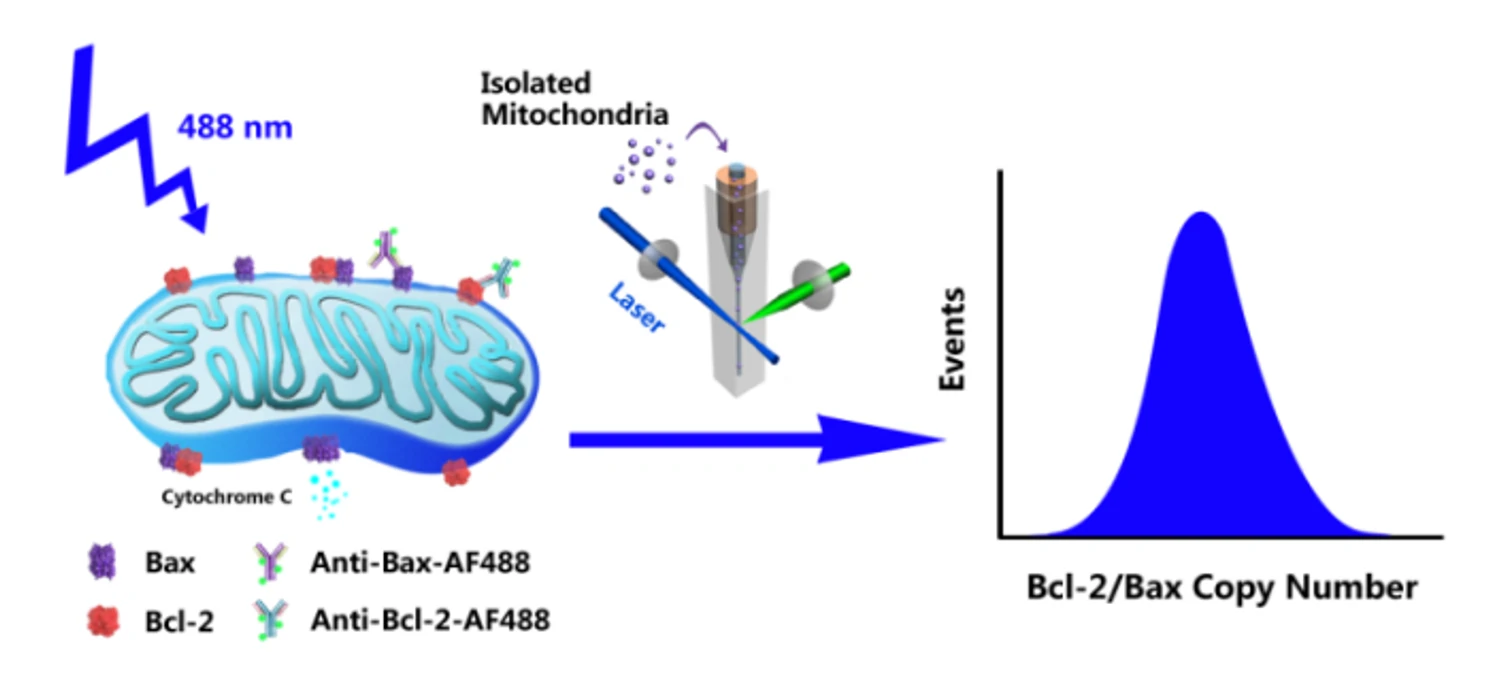

Figure 1. Quantitative measurement of Bcl-2 copy number in individual mitochondria.
A series of fluorescent nanospheres with fluorescence intensity calibrated by the unit of molecules of equivalent soluble fluorochrome (MESF)-AF488 were used to construct a calibration curve. The fluorescence intensity of a single mitochondrion could then be converted to the number of proteins per mitochondrion.
Biosens. Bioelectron., 2015, 74, 476-482.
Identification of Mitochondria-Targeting Anticancer Drugs and Mitochondrial Membrane Potential
Mitochondria play a pivotal role in determining the point-of-no-return in the apoptotic process. Therefore, anticancer drugs that directly target mitochondria hold great potential to evade resistance mechanisms that have developed toward conventional chemotherapeutics. Here, an in vitro strategy to quickly identify therapeutic agents that induce apoptosis by directly affecting mitochondria was reported. This result is achieved by treating isolated mitochondria with potential anticancer compounds, followed by simultaneously measuring the side scatter and mitochondrial membrane potential (ΔΨm, labeled with DiOC6(3)) fluorescence of individual mitochondria using the Flow NanoAnalyzer. The feasibility of this method was tested with eight widely used anticarcinogens. A potent mitochondrial membrane uncoupling agent, CCCP, was used as the positive control.
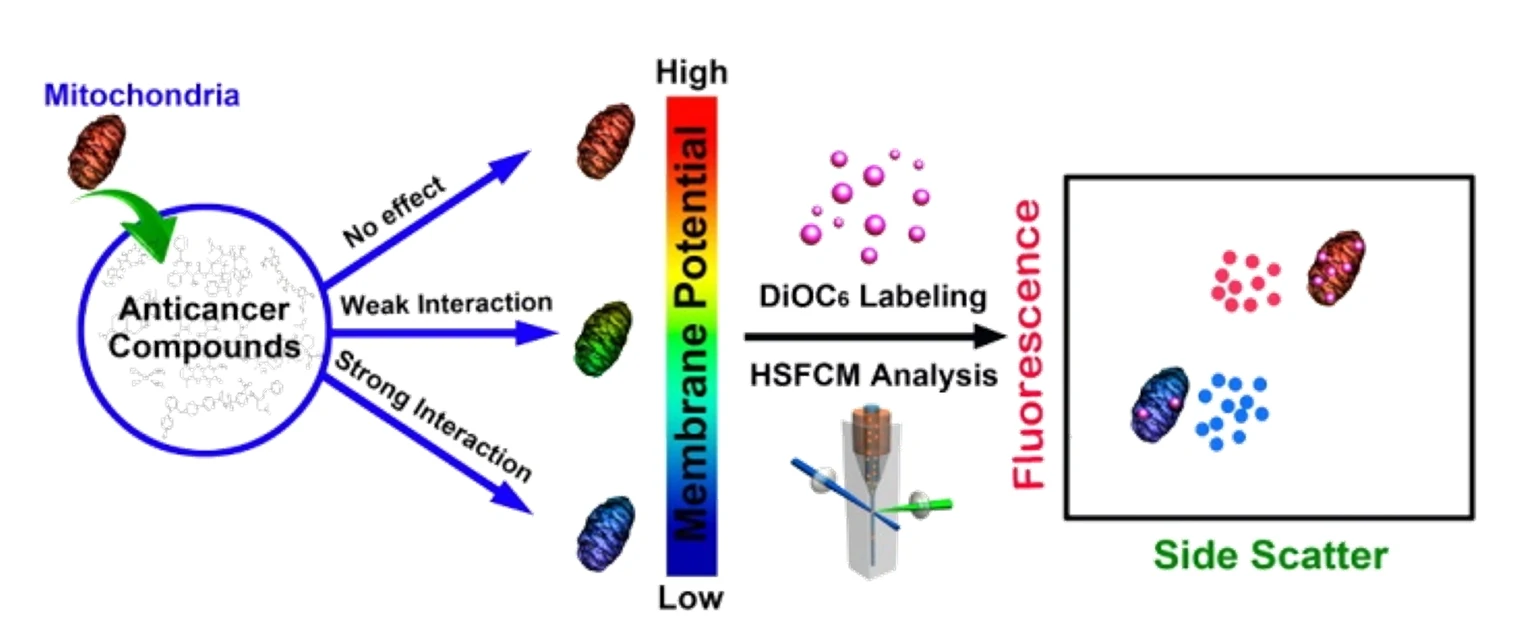
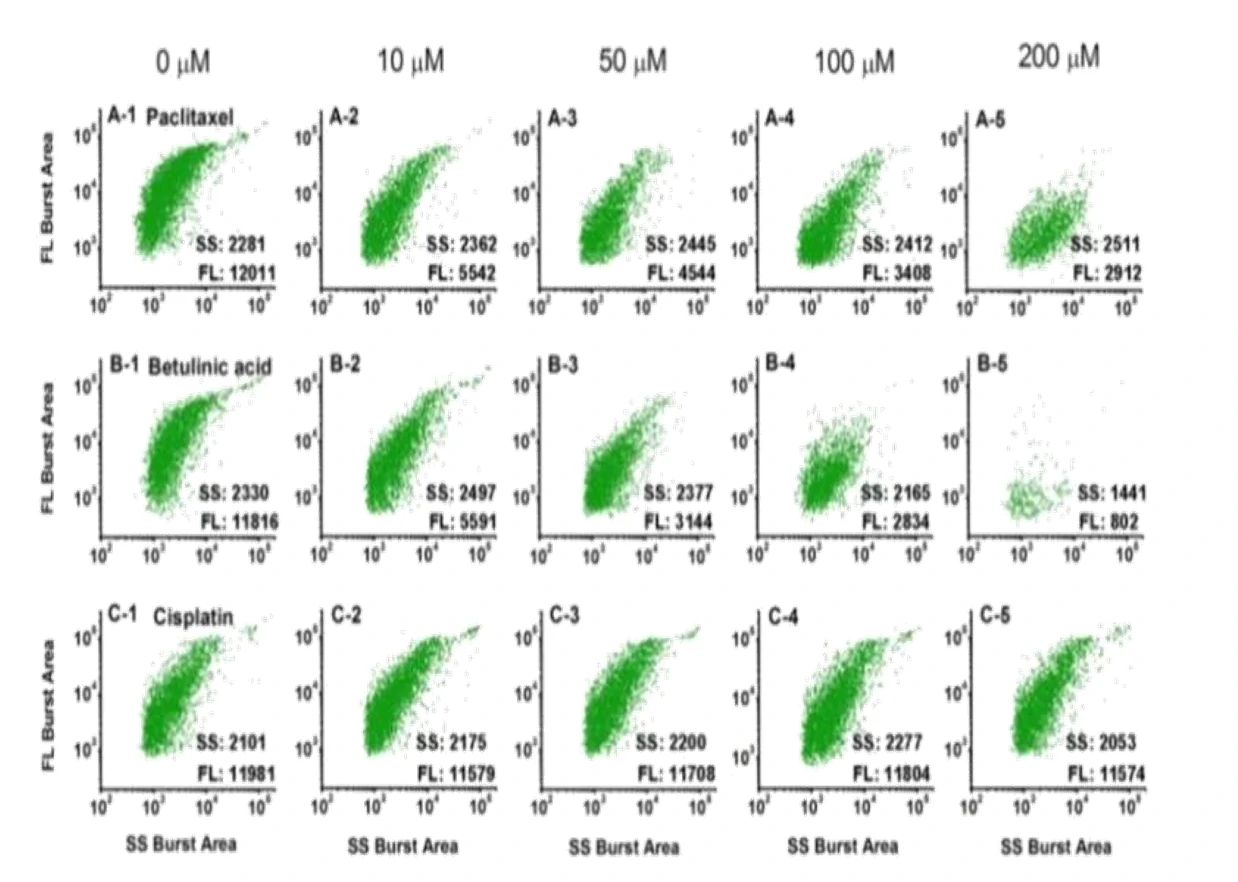
Figure 1. Analysis of mitochondria treated with different anticancer drugs on the Flow NanoAnalyzer.
By labeling with DiOC6(3), the Flow NanoAnalyzer can monitor the change of mitochondrial membrane potential and quickly and accurately detect whether the drug can act directly on mitochondria and its effect. This provides a new way for the detection of anticancer drugs’ mechanisms and screening.
Anal. Chem., 2014, 86(11), 5232-5237.
Mitochondrial Fusion and Apoptosis
Mitochondrial fusion is essential for maintaining genomic stability and physiological functions of mitochondria. Since mitochondrial fusion and fission work in synergy to regulate mitochondrial morphology and functions, it has been challenging to quantitatively measure the direct roles of mitochondrial fusion in apoptosis and cancer progression. Here, a high-throughput in vitro method to quantify mitochondrial fusion through single mitochondria analysis by the Flow NanoAnalyzer was reported.
Isolated mitochondria expressing green fluorescent protein (GFP-mito) or Discosoma red fluorescent protein (DsRed-mito) were mixed together, induced to fuse, and analyzed by the Flow NanoAnalyzer. An individual particle simultaneously exhibiting green and red fluorescence was identified as an event of heterotypic fusion, and the efficiency of heterotypic fusion was used as a surrogate for overall fusion efficiency. The as-developed method was applied to reveal the interplay between mitochondrial fusion and apoptosis without the interference of fission. The data suggests that disruption of mitochondrial fusion could be a potent strategy for cancer therapy. Furthermore, the as-developed method offers an effective approach to identify fusion inhibitors, including betulinic acid and antimycin A, providing reasons for their powerful utility in cancer treatment.
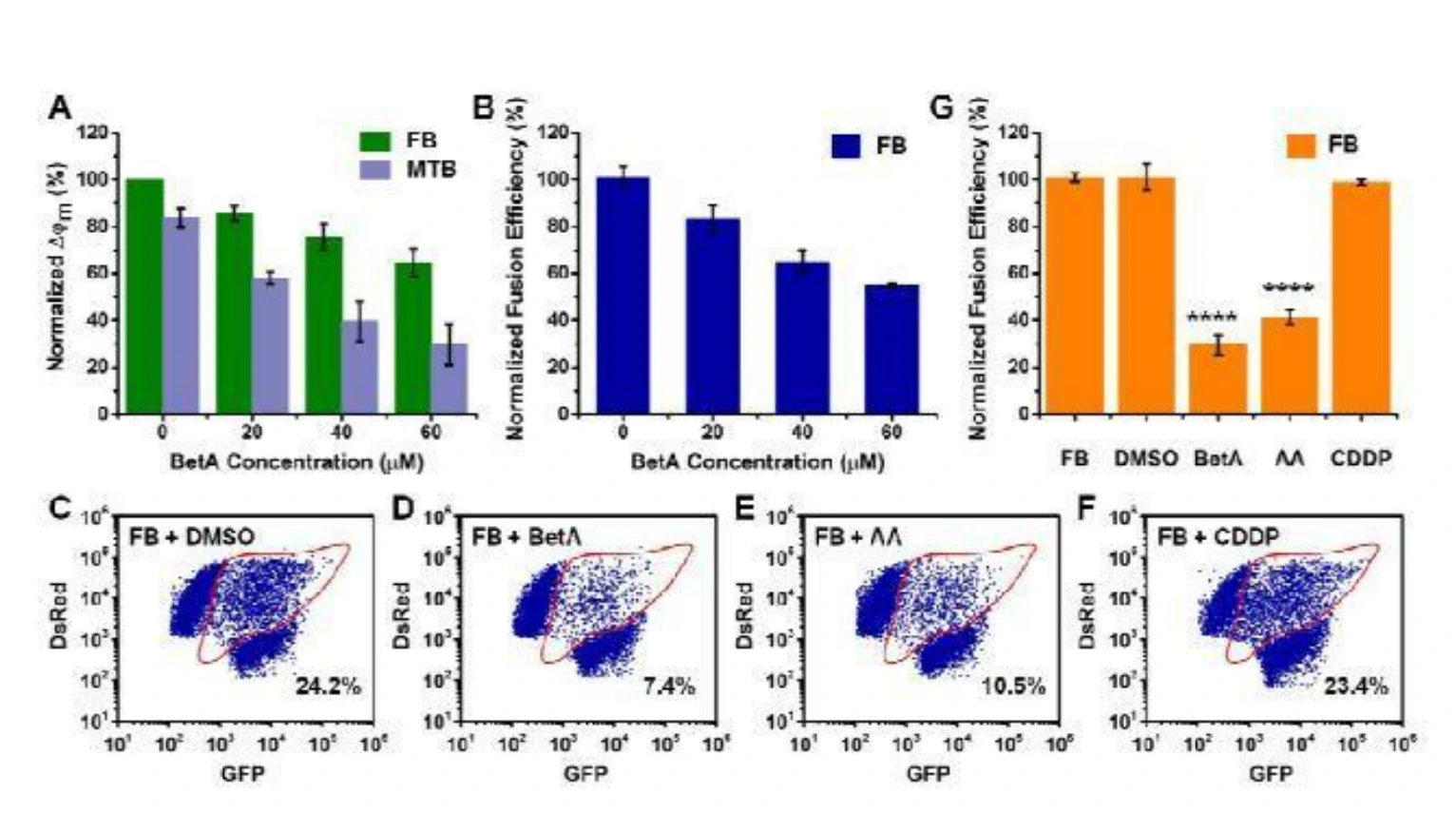
Figure 1. Analysis of the effect of mitochondrial fusion on cell apoptosis by the Flow NanoAnalyzer and comparison of the fusion effects of different compounds.
Fusion was promoted by cytosol derived from healthy cells but inhibited by cytosolic apoptotic pathways. Mitochondrial fusion could inhibit apoptosis by delaying the collapse of mitochondrial membrane potential. Thus, disruption of mitochondrial fusion could be an efficient strategy in cancer therapy.
Talanta, 2020, 222, 121523.