Extracellular Vesicles Encapsulated Oncolytic Viruses for the Treatment of Tumor Diseases
Extracellular vesicles possess the ability to evade the immune system and traverse the blood-brain barrier. They can also target tumor cells through specific markers, making them one of the most ideal carriers for drug treatment of tumor diseases. Oncolytic viruses (OAs) are tumor-killing viruses with replication ability, selectively infecting tumor cells by deactivating tumor suppressor genes in target cells, replicating in their cytoplasm, and eventually destroying them. They also stimulate the immune response, attracting more immune cells to continue killing residual cancer cells. However, due to the innate and antiviral immunity of the human body, oncolytic adenoviruses cannot reach specific tumor tissues, limiting their application in tumor treatment.
This study engineered extracellular vesicles with targeting functions and antiviral immunity, including the encapsulation of oncolytic adenovirus into extracellular vesicles. Exosome-encapsulated oncolytic adenovirus can resist the innate and acquired immunity of the human body. It can specifically infect tumor cells, self-replicate to form a large number of viruses that specifically target tumors, and subsequently kill tumor cells.
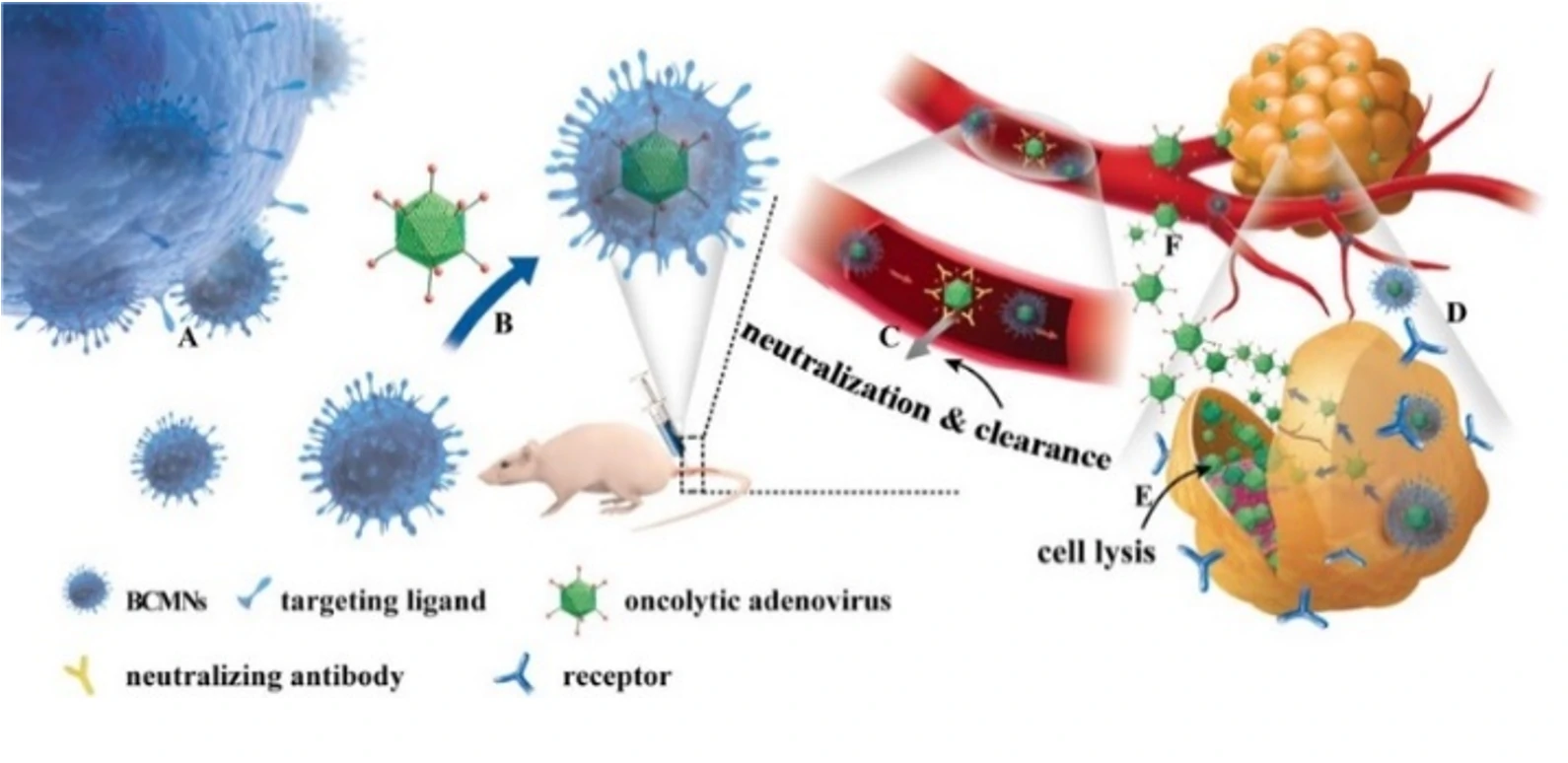
This study demonstrates that exosome-encapsulated oncolytic viruses exhibit antiviral immunity, and the targeting of tumor cells can be enhanced by labeling tumor-specific markers on the surface of exosomes. Releasing the oncolytic adenovirus into the exosomes is a key step in the experiment. The encapsulation efficiency and effectiveness of oncolytic adenovirus directly impacts the subsequent treatment outcomes. The researchers used fluorescent dye CFSE and nucleic acid dye SYTO™ 62 to label exosomes and OA, respectively. The Flow NanoAnalyzer was employed to evaluate the encapsulation efficiency of oncolytic adenovirus.
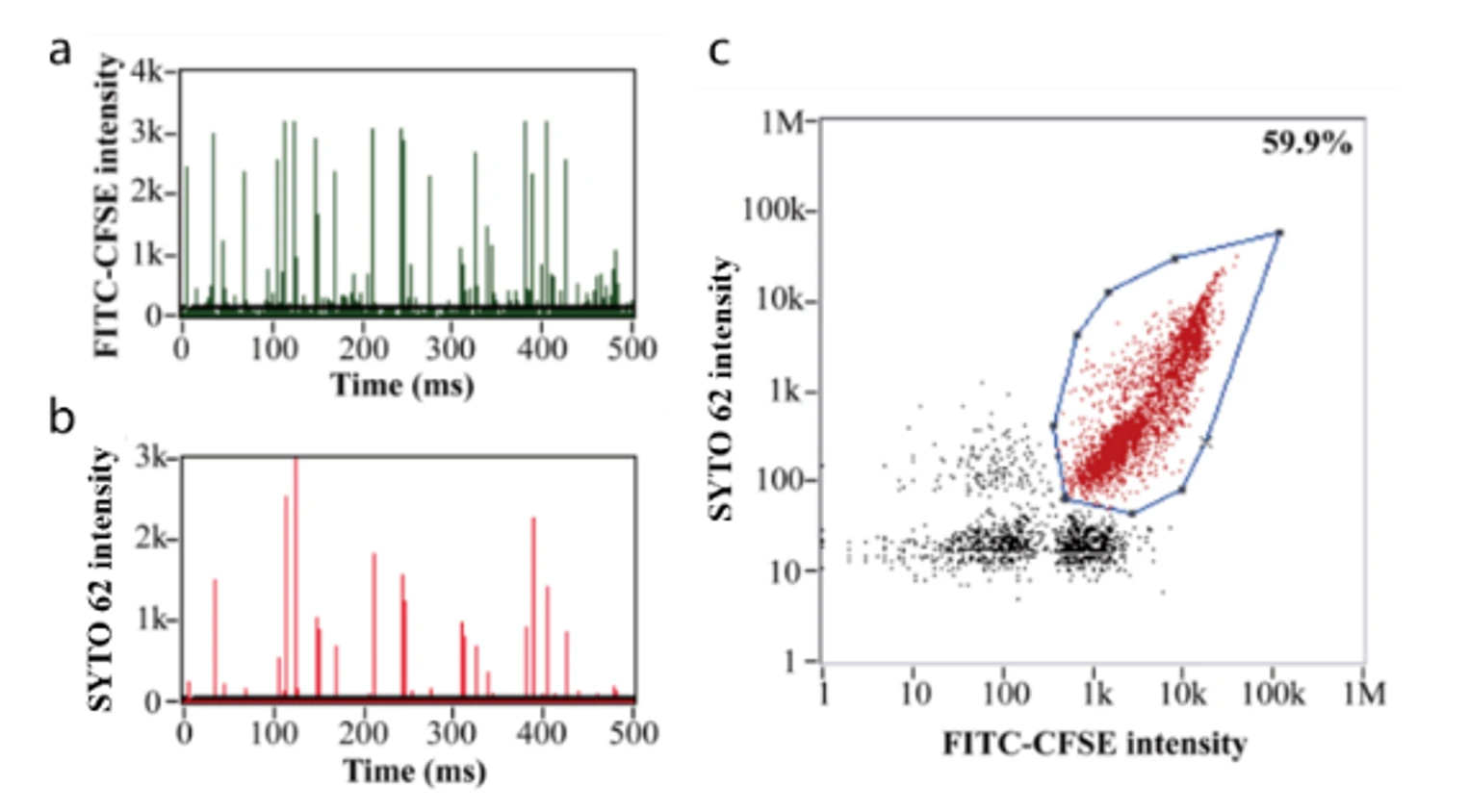
Figure 1. Encapsulation efficiency of oncolytic viruses.
Based on nucleic acid staining, the Flow NanoAnalyzer allows the determination of the encapsulation efficiency of oncolytic adenoviruses, which was 59.9% in this case.
Nano Lett., 2019, 19(5), 2993-3001.



