Applications in the Lentivirus industry
Research and Development
NanoFCM can achieve fast quantitative analysis of different components (mature virions、empty capsids、free viral nucleic acids) in the sample, which is essential to the optimization of plasmid transfection and screening of efficient and stable virus-producing cell lines enabling faster large-scale manufacture of lentivirus. The NanoFCM platform is very instructive for optimizing other parameters such as the type or amount of transfection reagents.
Production
Mass production of lentiviruses often involves suspension culture. In the culture process, attention is paid to the influence of cell culture density, speed of rotor, nutrient solution formulation, temperature, pH, harvest time and other conditions on the production efficiency of lentivirus. NanoFCM enables rapid analysis of virus samples within 2-3 min, monitoring the impact of these parameters on lentivirus production in real time and determining the optimal parameters in the virus production process. In contrast to the other traditional methods, NanoFCM can not only analyze purified lentivirus, but also be applied into measurements of complex samples during research and development. Such fast, real-time monitoring is very helpful for the optimization of lentivirus production.
Downstream process
By using NanoFCM, we can evaluate and compare different purification methods in order to determine the optimal combinations and workflow. In this way, NanoFCM can monitor each step of the purification process and its impact on recovery efficiency and the structural integrity of lentiviral products, identify critical steps which may result in industrial loss. By improving the elution time, ionic strength and other parameters, the determination of optimal purification techniques is possible.
Quality Control
NanoFCM can conduct rapid detection of the concentration, size, protein expression, stability, and ratio of mature virions, assessing the differences between different batches, and monitoring the stability of lentiviruses, so as to establish the standard for lentivirus production and storage.
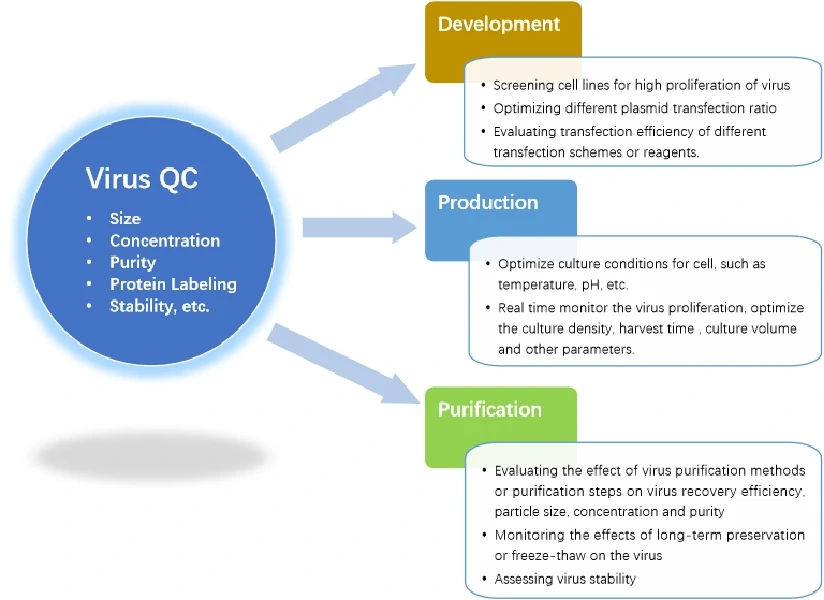
These are applications in the Lentivirus industry.




