NANOMEDICINE
COMPREHENSIVE MEASUREMENT SUITE FOR NANOSIZED DRUG PRODUCTS
Today, cancer is still a lethal illness around the world. As a cutting-edge discipline, nanotechnology, and more specifically, nanomedicine, is expected to provide solutions to the cancer problem. Nanomedicine is one kind of delivery systems in the nanometer size range, containing encapsulated, dispersed, adsorbed, or conjugated drugs and imaging agents. Drug delivery strategy has greatly promoted the treatment and application of drugs. The rapid development of drug therapy has benefitted from the continuous pursuit of progress in the delivery technology and strategy. Currently, nanomedicine can be broadly divided into the following categories: lipid nanoparticles (LNPs), polymeric nanoparticles, inorganic nanoparticles and virus nanoparticles. Among them, lipid based nanomedicine such as liposomes, solid lipid nanoparticles, lipid micelles and extracellular vesicles, which show the benefits of high biological compatibility and substantial drug-loading capability, are considered as the most successful drug delivery systems.
Nanoparticle-mediated gene therapy has received considerable attention over the past two decades. LNPs encapsulating siRNA is currently the most extensively clinically validated means of enabling RNA interference. As for the nucleic acid delivery, in addition to mRNA vaccines emerging in the COVID-19 epidemic, nucleic acid drugs also have a good prospect in tumor, chronic and rare diseases. However, it is challenging to determine the precise particle count and the fraction of loaded particles. Although, in principle, particles can be counted in cryo-TEM images, the nonuniformity of LNP distribution in vitrified samples on electron microscopy grids makes it difficult to obtain a precise particle count. Moreover, the comparable size, shape, and electron density of empty and siRNA-loaded LNPs render cryo-TEM less effective in determining the fraction of siRNA loading. The effect of a nanomedicine will be affected by its physical and chemical properties, including composition and properties of carrier materials, particle size and distribution, structure and morphology, affinity/hydrophobicity, particle concentration, drug loading, drug loading ratio, type and density of surface ligands, etc. Accurate characterization of various physical and chemical properties contributes to the design, development and quality control of nanomedicine. Sufficient quality control and highly-efficient drug delivery are vitally important. Taking advantage of the superior resolution, high sensitivity, fast detection speed and the capability of multi-parameter analysis of Flow NanoAnalyzer, a comprehensive characterization platform for nanomedicine has been developed, which allows the determination of particle size, drug content, drug encapsulation efficiency, particle concentration and the surface ligand density of nanomedicine at the single-particle level.
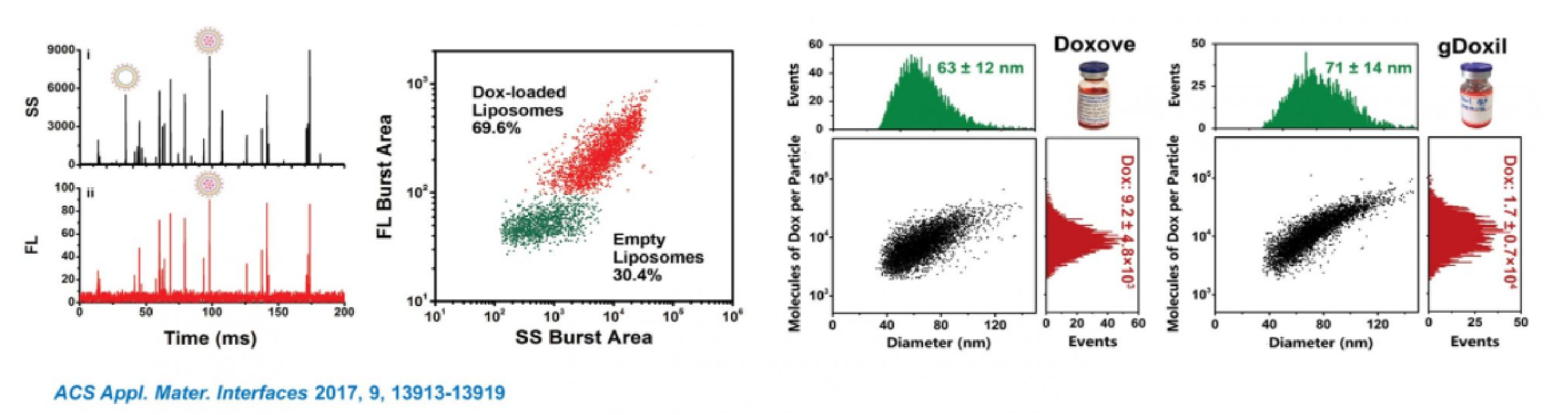
1. Characterization of Doxorubicin-Carrying Liposomes
Doxil (doxorubicin-carrying liposomes) is the first FDA-approved nanomedicine (1995), and DLS and cryo-TEM are the two most commonly used methods for size analysis. DLS is able to measure particle size for homogeneous samples. The characterization of particle size distribution of Doxil usually requires cryo-TEM and complex 3D reconstruction technology, which typically takes 2-3 days, and the obtained particle size distribution is not statistically representative. On the other hand, the efficiency of individual particles loaded with doxorubicin could not be assessed. Here, NanoFCM was used for rapid and simultaneous detection of scattered light and fluorescence signals of individual liposome particles. With the help of particle size standards, comprehensive characterization of Doxil particles, including size distribution, particle concentration and doxorubicin encapsulation efficiency was achieved in only 2-3 minutes. In addition, the fluorescence intensity is also quantitive, which relates to the number of drug molecules. The fluorescence intensity can further reveal the correlation between the particle size and the content of Doxil loaded in each particle.
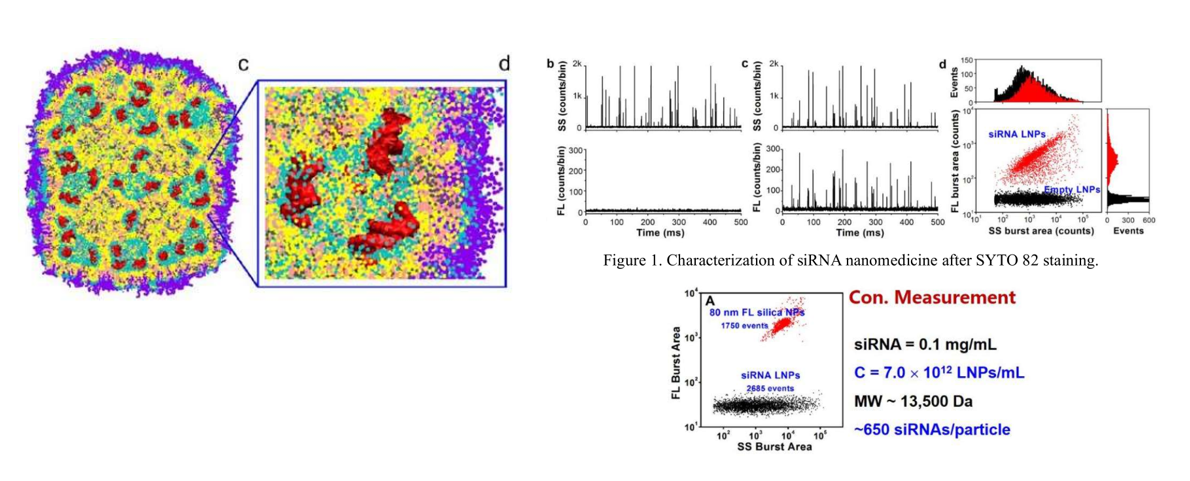
2. Lipid Nanoparticles for Targeted siRNA Delivery (Nucleic Acid Delivery)
Lipid nanoparticles (LNPs) encapsulating siRNA are currently the most extensively clinically validated means of enabling RNA interference. However, it is challenging to determine the precise particle count and the fraction of loaded particles. Although in principle, particles can be counted in cryo-TEM images, the nonuniformity of LNP distribution in vitrified samples on electron microscopy grids makes it difficult to obtain a precise particle count. Moreover, the comparable size, shape, and electron density of empty and siRNA-loaded LNPs render cryo-TEM less effective in determining the fraction of siRNA loading. In this study, Flow NanoAnalyzer is used for quantitative, multi-parameter characterization of siRNA loaded LNPs. Upon fluorescent staining with transmembrane nucleic acid dye SYTO 82, the fraction of siRNA loading is determined. The concentration of lipid nanoparticles can be quickly obtained by single particle counting, which can be used for concentration determination and copy number measurement of siRNA per lipid nanoparticle.
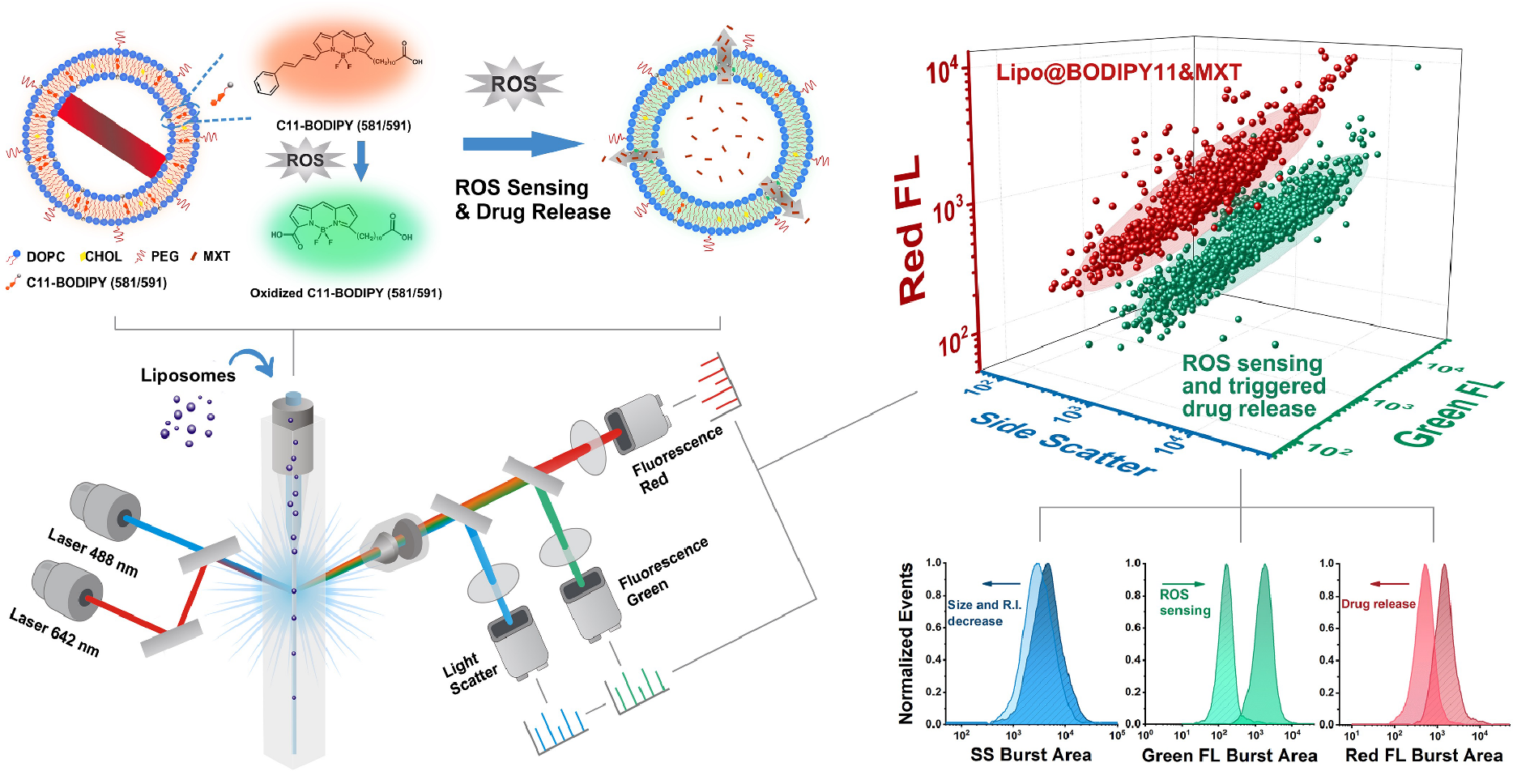
3. Theranostic Liposomes with Stimulus Sensing
C11-BODIPY (581/591) is a well established ratiometric fluorescent sensor for ROS detection in membranes. The emission peak shifts from 590 nm to 510 nm at the presence of ROS, which results in an increase of signal intensity in green FL channel, while decrease of signal intensity in red FL channel is observed. Here, fabricating a reactive oxygen species (ROS)-responsive liposome (Lipo@BODIPY11) and taking it as an example, a strategy for theranostic nanoparticle characterization by the Flow NanoAnalyzer was developed. The chemotherapy drug Mitoxantrone was encapsulated into C11-BODIPY functionalized liposomes, and the ROS sensing and drug release behaviors were simultaneously characterized by NanoFCM. The reduced intensity on SS and red FL along with the increase of green FL signal indicated a sustained drug release process at the elevated H2O2 concentration.
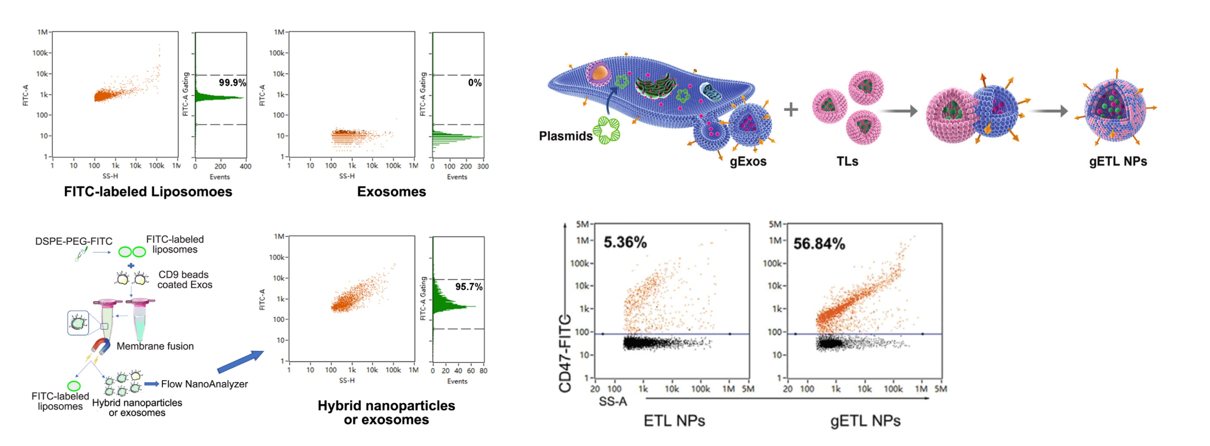
4. Liposome Fusion with Exosomes for Targeted Drug Delivery
Liposomes have long been used to encapsulate drugs, the synthesize and encapsulation procedures are simple and well-developed, it is easy to reach high encapsulation ratio. While CD47 on exosomes has proven to release “Don’t eat me” signal, and avoid to be removed by the immune system. The combination of liposomes and exosomes will be an ideal drug delivery platform. Here, thermosensitive liposomes encapsulated with drugs were fused with genetically engineered exosomes, the resulting exosome-liposome hybrid NPs (gETL NPs) display CD47 on the surface and bear thermosensitive agents inside. NanoFCM allows the analysis of liposomes and exosomes at single particle level, and the fusion efficiency is also determined. To confirm the success of fusion, liposomes were labeled with nitrobenzoxadiazole (NBD) through insertion of NBD-DSPE-PEG2000 and exosomes labeled with CD9 immunomagnetic beads through antigen-antibody reaction. After fusion, the gETL NPs were sorted out by a maget. Based on NBD fluorescence, the presence of liposome membrane was analyzed by Flow NanoAnalyzer, and the fusion efficiency was determined to be 95.7%. And the expression of CD47 on the gETL NPs (56.8%) generated from genetically engineered exosomes was much higher than that on ETL NPs (5.3%) generated by wild type exosomes, and enhanced efficiency of tumor cell phagocytosis was observed.