Applications in the Viruses industry
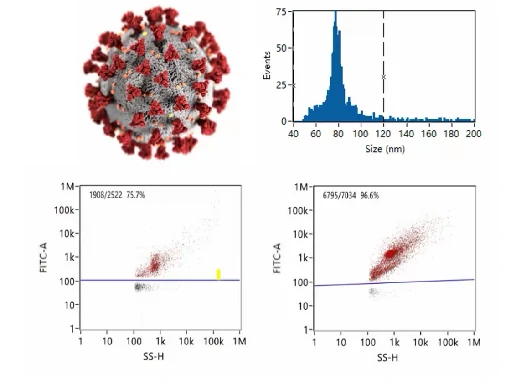
Quality Control of COVID-19 Vaccine
Quality control is indispensable to ensure the adequate purity of virus products in the biopharmaceutical industry. Purity assessment of virus products is indispensable in many biotechnology applications, especially in manufacturing vaccines. In the process of preparing virus products, many particles can coexist in the lysates of the host cells, including cell debris, empty capsids, and mature virions. What’s more, long-term tracking of the stabiilty is essential to maintain the high efficacy. Here a COVID-19 vaccine made from inactived Coronavirus is analyzed. The size (diameter) distribution and particle concentration of single novel Coronavirus could be acquired directly from the software of Flow NanoAnalyzer. The uniformity of viral particles is evaluatd directly, while purity can be assessed by nucleic acid staining. The purity of COVID-19 increased to nearly 100% after several rounds of purification steps. The Flow NanoAnalyzer platform enables quantitative and multiparameter analysis of single Coronavirus, which is distinctively sensitive, yet high-throughput, and shows great potential in quality control of COVID-19 vaccine.
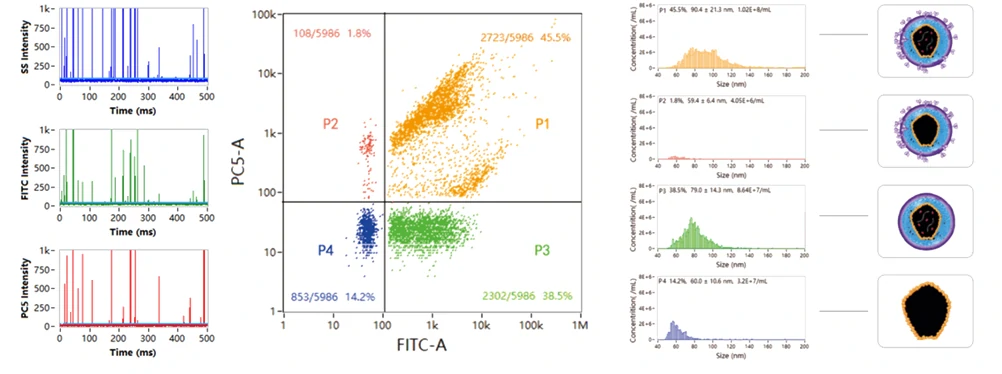
Comprehensive Analysis of Lentivirus for Cell and Gene Therapy
Chimeric antigen receptor (CAR) T cell Therapy for B cell malignancies is the first cell therapy approved by the US Food and Drug Administration for the treatment of cancer. After that, an increasing number of clinical trials are being conducted on CAR-T cell therapy. Lentivirus is the most widely used vector in CAR-T cell therapy. A complete CAR-T cell process includes the preparation of lentiviral vectors and the production of CAR-T cell products. Comprehensive characterization of lentiviruses is crucial to the successful construction of CAR T. The size distribution of lentivirus is determined through direct light scattering detection. Membrane permeable nucleic acid dye is used to label the RNA, while the VSV-G protein is specifically recognized by fluorescent-tagged monoclonal antibody. A particle with both RNA signal and VSV-G signal is identified as an effective lentivirus vector. Dual-label analysis of nucleic acid and protein of lentiviral vectors allows not only to optimize the culturing conditions and to maximize the production of the virus, but also to screen the virus strains that effectively express the capsule proteins.
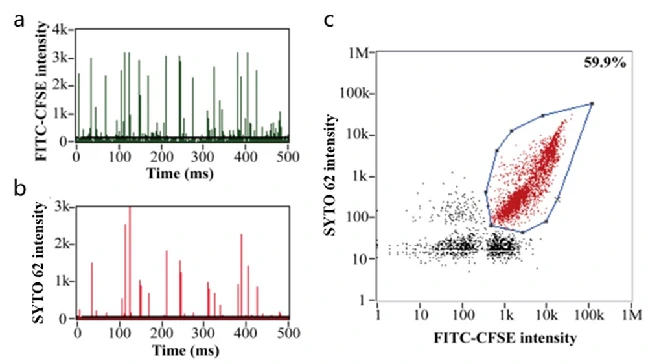
Oncolytic Adenovirus for Treatment of Tumor
Oncolytic Viruses are a type of tumor-killing virus with replication ability, which selectively infect tumor cells by deactivating tumor suppressor genes in target cells, replicating themselves in their cytoplasm and eventually destroying them. It also stimulates the immune response, attracting more immune cells to continue killing residual cancer cells. Newcastle disease virus, herpes simplex virus, reovirus and adenovirus have all been modified into oncolytic viruses due to their oncotropic properties, which have the function of specific recognition and lysis of tumor cells, while have no killing effect on normal somatic cells. Oncolytic Adenoviruses (OA) have been explored in both preclinical and clinical therapies. While the poor targeting delivery is a major obstacle that limits OA application. Here OA was decorated with bioengineered cell membrane nanovesicles (BCMNs) that are genetically engineered with targeting ligands, the resulting OA@BCMNs exhibited enhanced targeting delivery, vial oncolysis, and survial benefits in multiple xenograft models. Nano-flow cytometry was used to evaluate the encapsulation efficiency of OA@BCMNs, CFSE and SYTO 62 probes were applied to label the BCMNs and nucleic acids of OA, respectively. The positive ratio of 60% demonstrate the successful preparation of the OA-encapsulated BCMNs nanostructure.
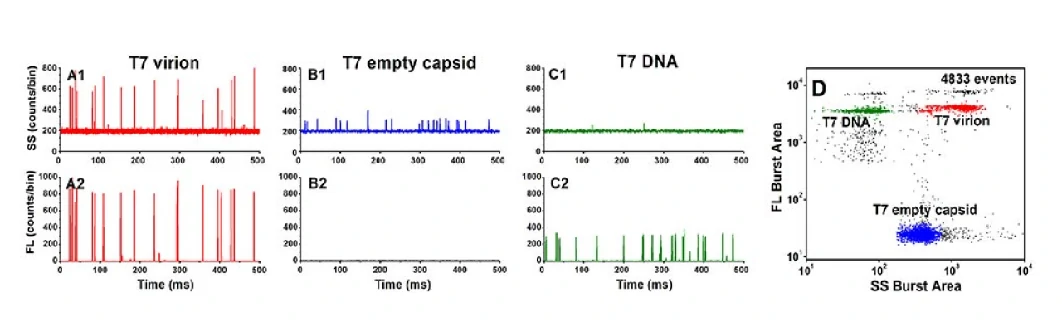
Virus-based drug delivery
Virus particles can be regarded as natural nucleic acid nanomaterials, which consist of nucleic acid (drugs) wrapped in an empty virus shell (drug carrier). Through nucleic acid staining, the complete virus, empty virus shell, free nucleic acid and other components can be quickly distinguished from the mixture, and further reveal the drug packaging efficiency, the amount of package, and the proportion of effective drugs and other parameters.
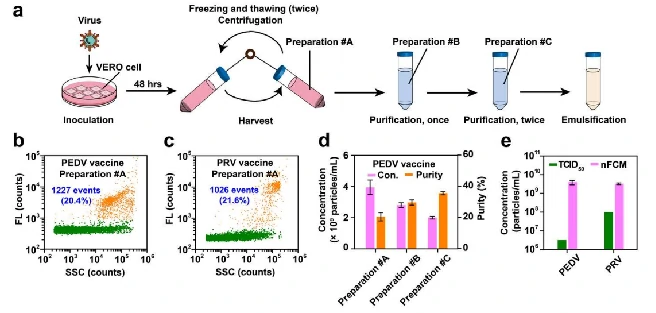
Viral Vaccines
The production process of viral vaccine includes virus culture, harvest, purification and emulsification. By nucleic acid staining, the Flow NanoAnalyzer can quickly assess the virus at different stages of production, providing information on particle concentration and purity. According to the experimental results,with the further purification process, the concentration of virus particles gradually decreased, but the purity showed an upward trend.
Purity Assessment of Adenovirues
Through side scattering detection and concurrent fluorescence detection by nucleic acid staining, purity analysis of adenoviruses is quickly achieved.

These all about Applications in the Viruses industry.




